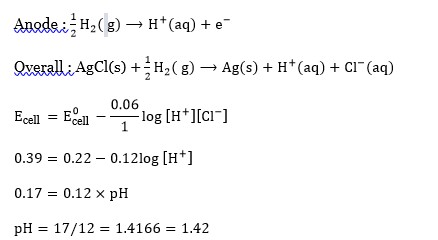Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 9
At room temperature water is liquid and has boiling point 373K due to hydrogen bonding. Where as H? S is gas and it has no hydrogen bonding. Hence boiling point of H? S is less than 300K [Boiling point of H? S is -60 °C ].
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 10
First, find the initial velocity (v) of the ball as it leaves the machine, using the kinematic equation for maximum height:
v² = u² + 2as ⇒ 0 = v² - 2gh_max
v² = 2gh_max = 2 * 10 * 20 = 400
v = 20 m/s.
Now, apply the work-energy theorem to the ball while it is being pushed by the machine. The work done by the constant force F equals the change in kinetic energy of the ball.
Work done W = F * d
Change in K.E. = (1/2)mv² - 0
F * 0.2 = (1/2) * 0.15 * (20)²
F * 0.2 = (1/2) * 0.15 * 400 = 0.15 * 200 = 30
F = 30 / 0.2 = 150 N.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers