Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Translational kinetic energy will be equal to rotational kinetic energy corresponds to each degree of freedom.
New answer posted
4 months agoContributor-Level 10
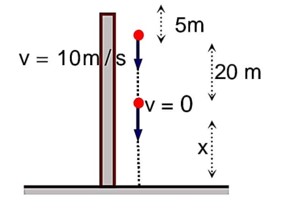
When second stone is released
Equation for first ball:
Equation for second ball :
Using these two equation
20 = 10t t = 2 sec
H = 20 + 20 + 5 = 45 m.
New answer posted
4 months agoContributor-Level 10
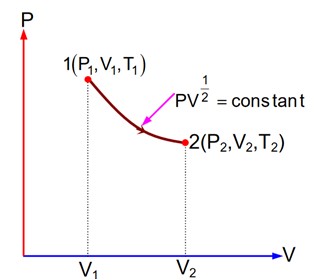
Since equation of the gas process is given so we can convert it into T & V form.
=
New answer posted
4 months agoContributor-Level 10
As we know that maximum acceleration will act on the particle when it is at extreme position .
New answer posted
4 months agoContributor-Level 10
The early 20th century experiments on electrical discharge through gases eventually led to the discovery of cathode rays (electrons). The major finding was that the characteristics of these cathode rays (electrons) were independent of the material used for the electrodes and the nature of the gas present in the cathode ray tube. This consistent behaviour across different substances led to the conclusion that electrons are a basic constituent of all atoms.
New answer posted
4 months agoContributor-Level 10
Before 20th century, atoms were widely considered the indivisible building blocks of matter. This view goes back to Indian and Greek philosophies, as old as 400 B.C. It also became one established thought on a scientific basis by John Dalton in 1808. With his theory, several fundamental laws of chemistry were established. But those laws failed against observations like static electricity. The experimental observations made towards the end of the 19th and beginning of the 20th century definitively proved that atoms are made of subatomic particles.
New answer posted
4 months agoContributor-Level 10
NH2CN (s) + O2 (g) ® N2 (g) + O2 (g) + H2O (l)
=
So, magnitude of
New answer posted
4 months agoContributor-Level 10
NaOH + Na2CO3
(1) When Hph is added
m.e NaOH + (m.e = milli equivalents)
(2) When MeOH is added after Hph
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers