Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
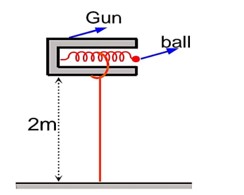
Let ball starts its motion with horizontal velocity v0, so with the help of conservation of mechanical energy, we can write
t = Time required to fall the ball =
New answer posted
5 months agoContributor-Level 10
The equation of wave at any time t will be
New answer posted
5 months agoContributor-Level 10
In the frame of vehicle, vehicle is in equilibrium under the influence of pseudo force FP
N = mg cos 30° + FP sin 30°
, and
By doing (1) * cos 30° - (2) * sin 30°, we have
New answer posted
5 months agoContributor-Level 10
Since binary mass system performs circular motion about is common centre of mass, so
Similarly we can show that
Hence their angular velocity will be same, time period will be same, i.e. TA = TB
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers