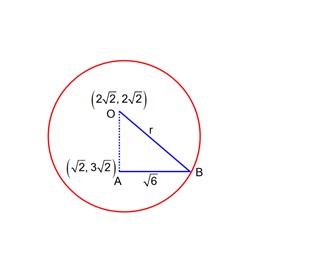
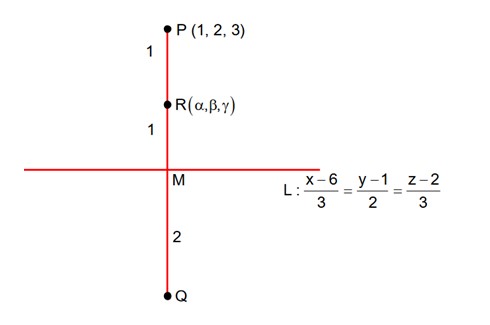
Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Let z be equal to (x + iy)
(x + iy) + (x – iy) = (x + iy)2 (i + 1)
Equating the real & in eg part.
(i) & (ii)
4xy = -2x Þ x = 0 or y =
(for x = 0, y = 0)
For y =
x2
x =
=
of
=
New answer posted
5 months agoContributor-Level 10
For this limit to be defined 2x3 – 7x2 + ax + b should also trend to 0 or x ® 1.
2 – 7 + (a + b) = 0
(a + b) = 5 …………….(i)
Now this becomes % form we apply L'lopital rule
Now the numerator again ® 0 as x = 1
6x2 – 14x + a ® 0 as x = 1
6 . (1)2 – 14 + a = 0
a = 8 …………….(ii)
a + b = 5
(b = -3) ® from (i) & (ii)
New answer posted
5 months agoContributor-Level 10
= 140……………… (i)
If any one student get less 50 marks then
but
both condition cannot satisfy together hence no students fails.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers