Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: As per the Hund's rule the half filled and fully filled orbital leads to the extra stability due to the symmetry thus fully filled 3d and half filled 4s is preferred.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:

We know that energy is inversely proportional to the wavelength

Thus, the increasing order of energy is B
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The mass number (A) is defined as the sum of the number of protons and neutrons present in the nucleus and the atomic number is defined as the number of protons or electrons present in an atom. Thus A=13, the number of neutrons is 7 so the number of protons is 6. Thus, the atomic number is 6.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The charged particles get deflected by the electric field. As among the given option only neutron is the particle that is neutral thus it does not get deflected by the electric field.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
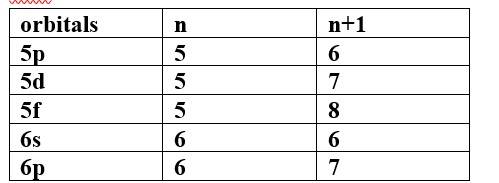
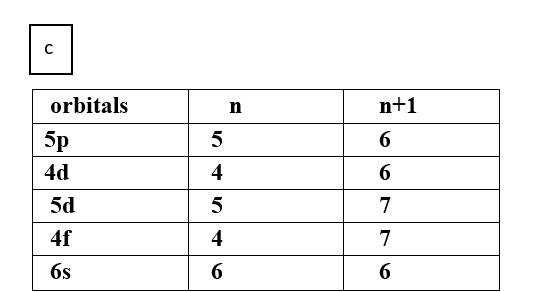
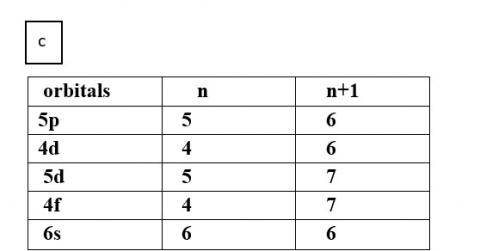
Ans: Based upon the above information, arrange the following orbitals in the increasing order of energy.
(a) 1s, 2s, 3s, 2p
(b) 4s, 3s, 3p, 4d
(c) 5p, 4d, 5d, 4f, 6s
(d) 5f, 6d, 7s, 7p
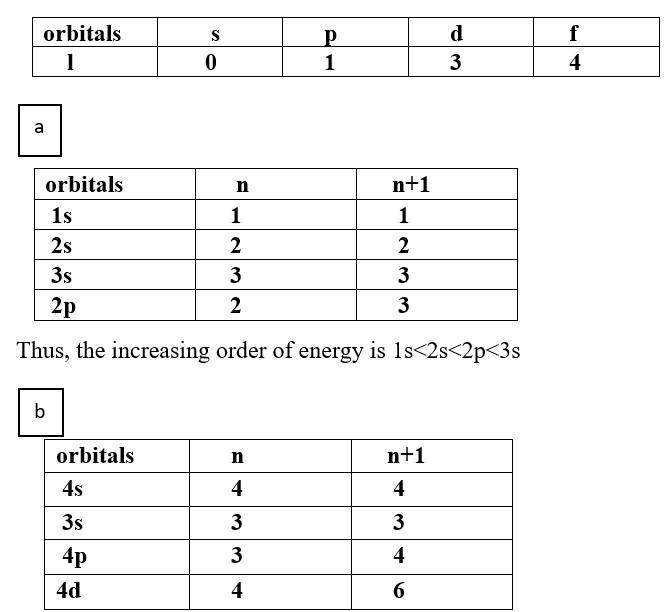
Thus, the increasing order of energy is 3s<3p<4s<4d
Thus, the increasing order of energy is 4d<5p<6s<4f<5d
Thus, the increasing order of energy is 7s<5f<6d<7p
(II) Based upon the above information, solve the questions given below :
(a) Which of the following orbitals has the lowest energy?
4d, 4f, 5s, 5p
Ans -
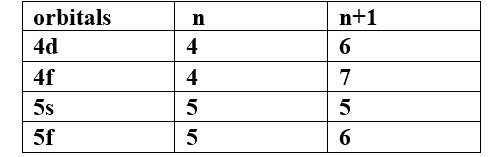
The lowest
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The number of radial nodes is given by n-l-1, where n is principal quantum number, l is azimuthal quantum number. The number of angular nodes is given by n-l, where n is principal quantum number, l is azimuthal quantum number. Here n =3 and l =1 Thus, angular nodes = 3-1 = 2 and radial node = 3-1-1 = 1.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: In a multielectron atomic system the energy of an electron depends not only on its principal quantum number (shell), but also on its azimuthal quantum number (subshell). Electrons having the same shells and same subshells have the same energy and they are known as degenerate orbitals.
Thus 3dxy ,3dz2 , 3dyz and 4dxy, 4dyz, 4dz2 are degenerate.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The electronic configuration of nickel is 1s2 2s2 2p6 3s2 3p6 3d8 4s2 , thus it loses electrons from its 4s orbital as its energy is higher compared to the other orbital.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The electronic configuration of oxygen atom is 1s2 2s2 2p4 and its orbital diagram is given by

New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The effective nuclear charge (Zeff) is defined as the net positive charge experienced by the outermost electrons in the atom. With the increase of Azimuthal quantum number (l) the Zeff experienced by the electron decreases. Hence the arrangement of subshells in the increasing order of Zeff is : d
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers