Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Complete combustion of compound produces 0.2 gm CO2
Hence wt of carbon in 0.2 gm CO2
Therefore % of carbon in compound
New answer posted
3 months agoContributor-Level 10
Here, total meq of acetic acid = 50 * 0.1 = 5
And total meq of NaOH = 25 * 0.1 = 2.5
After neutralization process
Meq of left acetic acid = 2.5
And meq of formed CH3COONa = 2.5
New answer posted
3 months agoContributor-Level 10
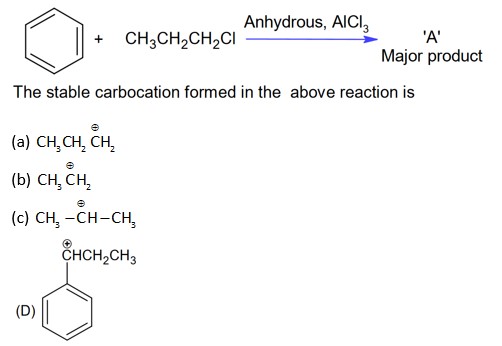
With AlCl3, alkyl halide will form cabocation which will show rearrangement.
New answer posted
3 months agoContributor-Level 10
In this carbocation +M effect of -OCH3 group stabilizes the carbocation.
While in option (A) and (B), +M of -OCH3 will not work but in option (C), +M of -OCH3 works so due to more delocalization in option (D), it is more stable.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers