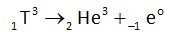Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Tritium is radioactive and it decays into He3 during emission of b-radiation
New answer posted
3 months agoContributor-Level 10
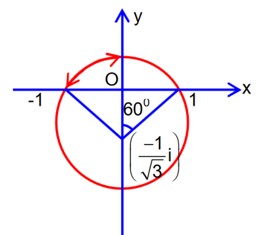
circle with radius = 3
arg part of a circle (with radius ). no common points
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 9
It happens in reversible reactions when the rate of the forward reaction becomes equal to the rate of the backward reaction. Result in the same concentration of reactants and product.
New answer posted
3 months agoContributor-Level 9
If the conditions of equilibrium are changed, it shifts to oppose the change. For example, in Haber's process, high pressure favors NH? formation.
New answer posted
3 months agoContributor-Level 9
The equilibrium constant is the ratio of the concentrations of products to reactants, each raised to the power of their stoichiometric coefficients. For reversible reactions.
New answer posted
3 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers