Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Due to high crystallity Be has the highest M.P.
Be = 1560 K
Mg = 925 K
Ca = 1120 K
Sr = 1062 K
New answer posted
3 months agoContributor-Level 10
During the electrolysis of dilute H2SO4
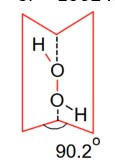
In the solid form of dihedral angle is equal to 90.2°.
New answer posted
3 months agoContributor-Level 10
In boron family lower O.S (+1) is more stabilize down the group due to inert pair effect.
New answer posted
3 months agoContributor-Level 10
Radius of Bohr's orbit
Radius of Bohr's orbit for hydrogen,
For third orbit (R3) = = r3 and
Fourth orbit (R4) =
New answer posted
3 months agoContributor-Level 10
Wt of liquid = 135 – 40 = 95 gm
Volume of liquid =
Hence volume of vessel = 100 ml = 0.1 lit from ideal gas equation,
New answer posted
3 months agoContributor-Level 10
Since velocity does not change, so acceleration will be zero.
mg = FB + Fv
New answer posted
3 months agoContributor-Level 10
Least count of Vernier = 0.1mm
Reading of Vernier Scale = 5 * 0.1 = 0.5mm
The corrected diameter of sphere = Main Scale Reading + Vernier Scale reading + Zero correction = 1.7 + 0.05 + 0.05 = 1.8cm = 180 * 10-2 cm.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers