Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
. [XeF? ]? has 7 electron pairs (5 bonding, 2 lone), pentagonal planar. XeO? F? has 5 electron pairs (trigonal bipyramidal).
New answer posted
4 months agoContributor-Level 9
Sol. (x/m) = k (P)¹/?
log (x/m) = logk + 1/n logP
Slope = 1/n = 2 So n = 1/2
Intercept ⇒ logk = 0.477 So k = Antilog (0.477) = 3
So (x/m) = k (P)¹/? = 3² = 48
New answer posted
4 months agoContributor-Level 9
The oxidation states of iron in these compounds will be
A = +2
B = +4
C = 0
The sum of oxidation states will be = 6.
New answer posted
4 months agoContributor-Level 9
Sol. E? cell = E? (Sn²? |Sn) - E? (Cu²? |Cu)
= -0.16 - 0.34 = -0.50V
ΔG? = -nFE? cell
= -2 * 96500 * (-0.5) = 96500 J
= 96.5 kJ = 96500 J
New answer posted
4 months agoContributor-Level 10
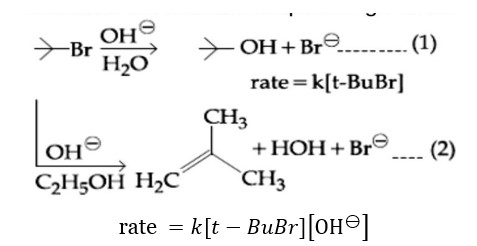
Reaction (1) is SN1 (rate independent of [OH? ]). Reaction (2) is E2 (rate depends on [OH? ]).
Statement (B) is correct. Changing concentration of base will have no effect on reaction (1).
New answer posted
4 months agoContributor-Level 9
Cesium has lowest ionisation enthalpy and hence it can show photoelectric effect to the maximum extent hence it is used in photo electric cell.
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 10
P = E/t
= 2/235 * (6.023*10²? *200*1.6*10? ¹? )/ (30*24*60*60) = 60W
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers