Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (a)
In Hoffmann bromamide degradation reaction as in this reaction primary amide group is treated with halogen first bromine then the halogen substituted amide product is coverts to primary amine with the release of carbon dioxide gas. Both the statements assertion and reason are incorrect therefore the correct option is a.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
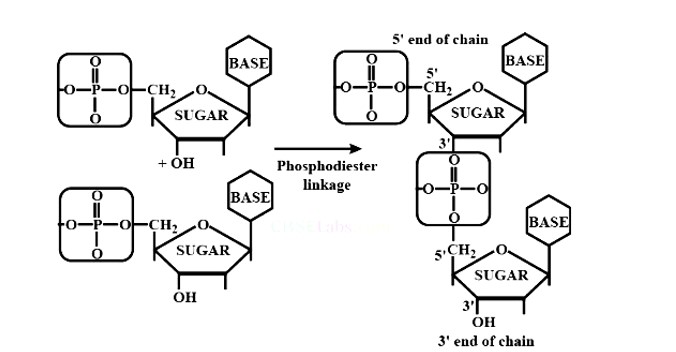
Nucleosides are linked to phosphoric acid at the 5′ position of the sugar moiety to produce a nucleotide. Furthermore, phosphodiester linkage between the 5′ and 3′ carbon atoms of pentose sugar bonds nucleotides (two molecules) together to generate dinucleotide. Phosphoric acid aids in the formation of this connection.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (c)
In the acryl derivative, the delocalisation of electrons of the nitrogen atom occur over the carbonyl group, this decreases the electron density on the nitrogen atom that it no more perform as nucleophile and don't react with next acylating alkaline molecule.
Therefore, Assertion statement is correct but reason is incorrect.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
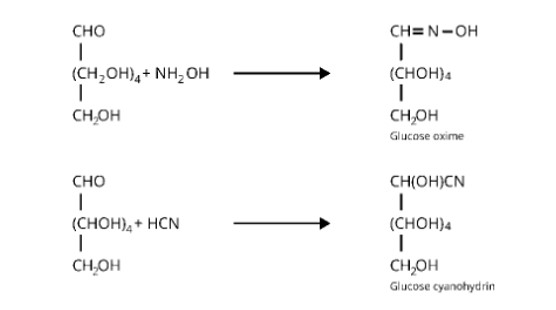
Glucose reacts with hydroxylamine to form a monoxime, which is then combined with one molecule of hydrogen cyanide to form cyanohydrin. The reactions are shown in the table below.
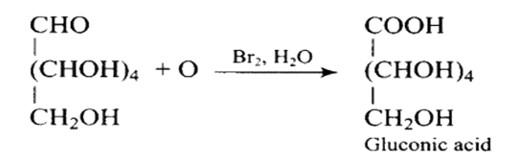
It has a carbonyl group as a consequence, which can be an aldehyde or a ketone. Gluconic acid, a carboxylic acid containing six carbon atoms, is formed when glucose is gently oxidised with bromine water.
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
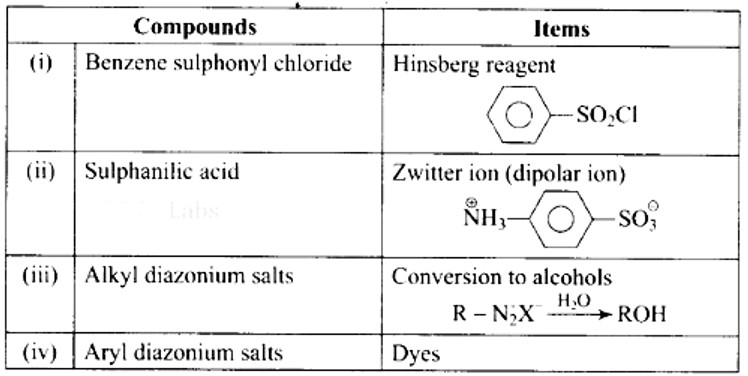
Ans: (i)- (b), (ii)- (a), (iii)- (d), (iv)- (c)
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Biocatalysts are enzymes that act as catalysts in biological reactions. They reduce the size of activation energy by finding a suitable path. Because the enzyme sucrase reduces the activation energy of sucrose hydrolysis from to, enzyme-catalyzed reactions occur at a much faster rate than traditionally catalysed ones.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Physical or chemical changes damage hydrogen bonding and other attractive factors. In addition, globules uncoil and the helix uncoils, resulting in a thread-like molecule. As a result, the biological activity of protein secondary and tertiary structures is lost entirely or partially. Protein denaturation is the term for this.
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
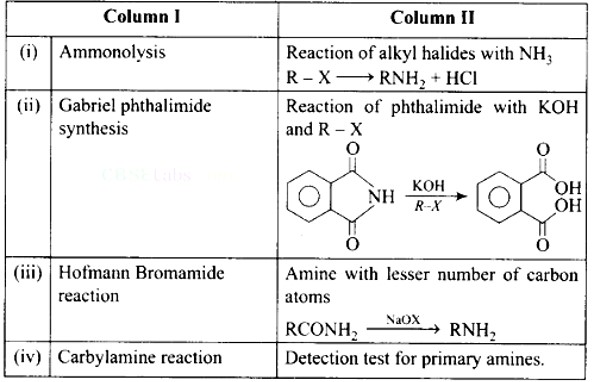
Ans: (i)- (d), (ii)- (c), (iii)- (a), (iv)- (b)
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Since both acidic (−COOH) and basic (−NH2) groups present in amino acids, it act like salts rather than simple amines or carboxylic acids. In Ans, a −COOH a group can release a proton and an amine group can take a proton, resulting in the formation of a dipolar ion known as zwitterion.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers