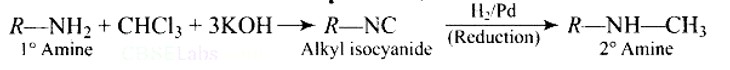Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: Carbylamine reaction is shown by primary amines only, primary amines react with chloroform in the presence of alcoholic KOH to form isocyanides which upon catalytic reduction gives secondary amines as the final product.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Medicines are used in diagnosis, prevention and treatment of diseases.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: Z is an aliphatic amine that produces a solid that is insoluble in base. This means that the reaction with C6H5SO2Cl must produce a product with no replaceable hydrogen attached to nitrogen.
To put it another way, the amine must be a secondary amine.
Z stands for ethylmethylamine.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The average molecular mass of drugs is of the order of 100-500 u.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
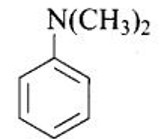
Ans: N, N-dimethylbenzenanmine
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
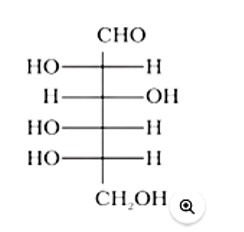
In the above given structure, the hydroxyl group (−OH) present at the last chiral carbon atom of the molecule, that is, C5, is directed towards the left. As a result, the given compound has an ' L '- configuration.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: CH3CH2CH3 < CH3CH2NH2 < CH3CH2OH
As oxygen is more electronegative than nitrogen therefore, the O-H bond is more polar than the N-H bond. So ethanol has more dipole moments than ethylamine. Propane is non- polar in nature hence, had the least among all.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a, c, d)
(a) Cationic detergents are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions. These detergents have germicidal properties.
(b) Bacteria cannot degrade the detergents containing highly branched chains, therefore, in most of the detergents used these days, the branching is kept to a minimum so that the detergents become easily biodegradable.
(c) Some synthetic detergents can give foam even in ice cold water.
(d) Synthetic detergents are cleansing agents which have all the properties of soaps, but which actually do not contain any
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: CS2 is a non-polar solvent which decreases the activating effect of? NH2. As a result, mono substitution occurs only at o- and p- positions giving a mixture of 2-bromoaniline (minor) and 4-bromoaniline (major) as products.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers