Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Sucrose acts as a dextrorotatory molecule. It produces a mixture of glucose and fructose when hydrolyzed, with particular rotations of +52.5? and −92.4? The net resultant mixture is laevorotatory because fructose laevorotation is greater than glucose dextrorotation. The sign of rotation shifts from dextro to laevo as a result of sucrose hydrolysis, and the resulting compound is known as invert sugar.
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Excess vitamin C is easily removed in the urine since it is water soluble. As a result, it can't be stored in the body and must be ingested frequently.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
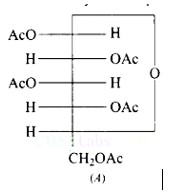
The given structure A is glucose pentaacetate, which lacks a free hydroxyl? OH or carbon -CHO group. As a result, it cannot be transferred to the open chain form to make a free group, and therefore the oxime is not produced.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
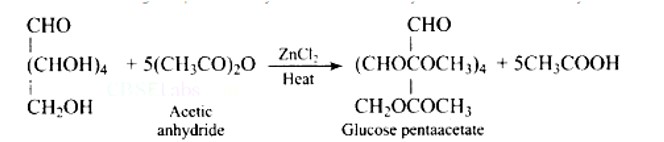
When glucose is acetylated with acetic anhydride, a penta-acetyl derivative is produced. The reaction is depicted in the diagram below.
This confirms the presence of five -OH groups.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
During the curdling process, the lactose sugar in milk is converted to lactic acid.
Milk curdling is caused by lactic acid-producing bacteria found in milk. Protein denaturation is an example. When a protein is exposed to physical or chemical alterations, it is said to be modified. The hydrogen bond has been shattered. Globules open up, helices uncoil, and proteins lose their biological function.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
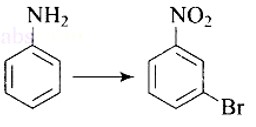
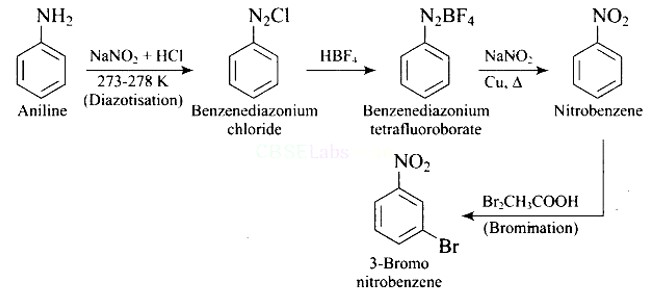
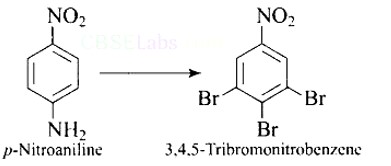
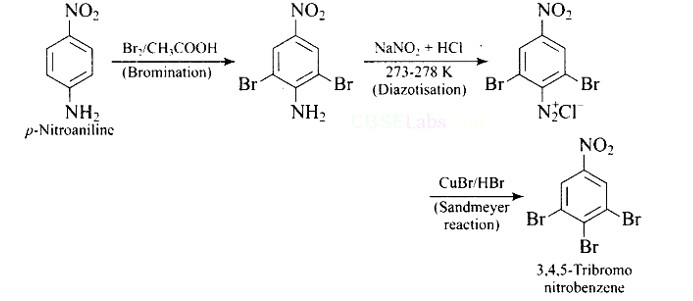
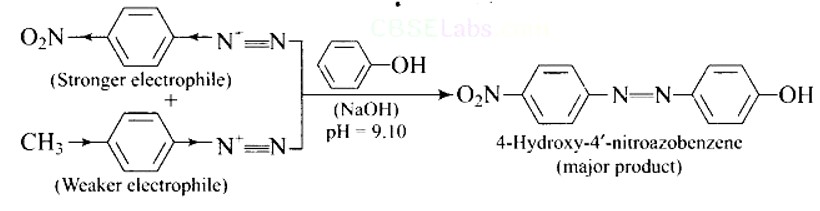
This is an example of an electrophilic aromatic substitution reaction. phenol generates phenoxide ion in alkaline medium, which is more electron-rich than phenol and thus more reactive to electrophilic attack. In this reaction, the electrophile is an aryldiazonium cation.
The faster the reaction, the stronger the electrophile.
The cation p-nitrophenyldiazonium is a stronger electrophile than the cation p-toluene diazonium.
As a result, it preferentially couples with phenol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers