Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a, d) Tranquilizers are neurologically active drugs. Veronal and luminal are derivatives of barbituric acid used as tranquilizers.
New answer posted
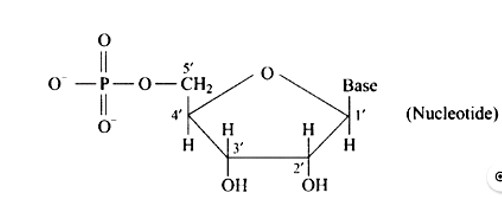
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The sugar moiety of a nucleoside is linked to a phosphoric acid unit, ideally at the 5? position, to form a nucleotide.
The structure is depicted in the diagram below.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a, d) Ranitidine and cimetidine are antihistamines which are used as ant-acids. These drug result in release of lesser amount of acid. Brompheniramine and terfenadine are antihistamines which act as antiallergic in drugs.
New answer posted
7 months agoContributor-Level 10
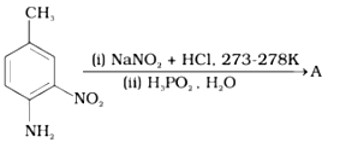
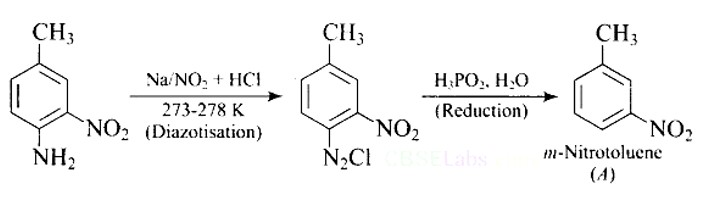
This is a Short Type Questions as classified in NCERT Exemplar
Ans: The best reagents for the conversion of nitrile to primary amine are LiAlH4 and Sodium/Alcohol. By reduction, the nitriles can be converted into a corresponding primary amine.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b, d) Sodium Hydrogen carbonate and Magnism Hydroxide, both are mild alkalies, are used as ant-acids.
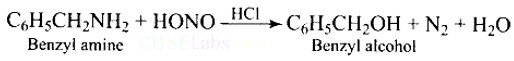
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: When Benzyl amine is treated with nitrous acid, firstly BDC is formed which is unstable and decomposes to Benzyl alcohol.
New answer posted
7 months agoContributor-Level 10
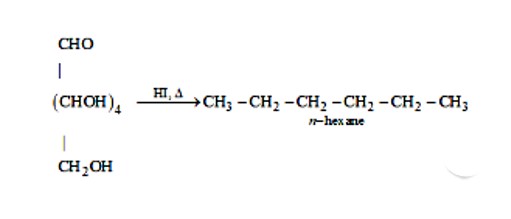
This is a Short Answer Type Questions as classified in NCERT Exemplar
n-hexane is produced when glucose is cooked with HI for a lengthy amount of time. The reaction is depicted in the diagram below.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c, d) Penicillin destroys bacteria by destroying the cell wall of the microorganism or kill the bacteria so, it has bacteriocidal effect. Penicillin has a narrow or limited spectrum.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
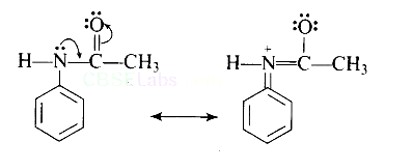
Direct nitration of aniline is not possible on account of oxidation of -NH2 group. However, nitration can be carried after protecting the -NH2 group by acetylation to give acetanilide which is then nitrated and finally hydrolysed to give o- and p-nitroanilines.
The acetyl group being electron withdrawing attracts the lone pair of electrons of the N-atom towards carbonyl group.
As a result, the activating effect -NH2 group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, acti
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers