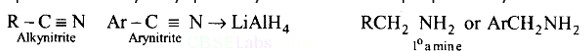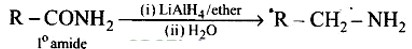Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct options are b and d.
Amylopectin and Glycogen are the correct choices since they are carbohydrate storage forms found in animal tissues, whereas amylose is made up of 80-85% starch. It's also a branched glucose polymer that branches via C1 - C6 glycosidic linkage.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A, B & C)
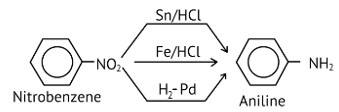
Nitro compounds are reduced to amines by passing hydrogen gas through an acidic medium containing finely divided nickel, palladium, or platinum, as well as by reduction with metals in an acidic medium.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct options are a and c.
Globular protein is a protein structure that forms when a chain of polypeptides coils around to produce a spherical shape. Insulin and albumin are two examples of water-soluble globular proteins. As a result, (a) and (c) are the correct answers.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct options are b and d.
Sucrose is a common disaccharide that breaks down into an equimolar mixture of D- (+)- glucose and D- (-)- fructose when hydrolyzed. These two monosaccharides are held together by a glycosidic bond between C1 of α - glucose and C2 of β fructose. Because the reducing groups of glucose and fructose are involved in the formation of glycosidic linkages, sucrose is a non-reducing sugar.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C and D)
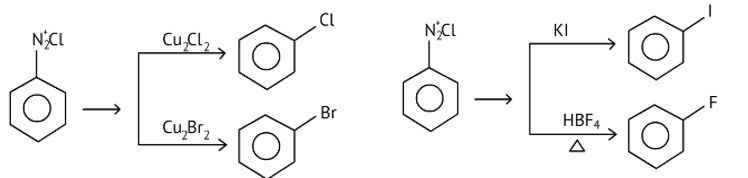
Chlorobenzene and bromobenzene are prepared by the sandmeyer's reaction.
While fluorobenzene and iodobenzene are prepared by simple heating of diazonium salt with aqueous KI Solution.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct option is C.
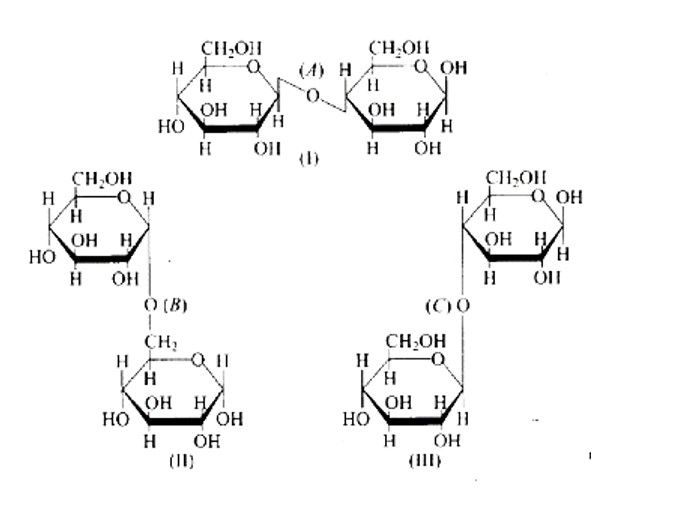
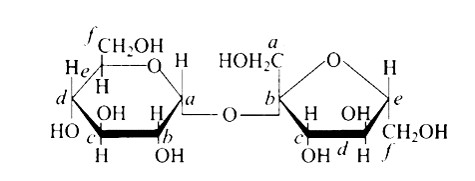
The links between C1 and C4 of glucose are shown as 'A' and ' C, ' respectively, whilst the linkage between C1 and C6 of the glucose units is shown as ' B. ' Furthermore, option (c) is clearly correct based on the structures.
Because ' B ' is the bond or link between the glucose units of C1 and C6 and, option (b) is wrong.
Option (a) is wrong because the relationship between C1 and C4 and is represented by 'C.'
Because 'A' is the hyperlink between C1 and C4 the glucose units of and, option (d) is wrong.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Aliphatic and arylalkyl primary amines can be prepared by the reduction of the corresponding nitriles with LiAlH4
Heating alkyl halide with primary, secondary and tertiary amine can be prepared by reduction of LiAlH4 followed by treatment with water.
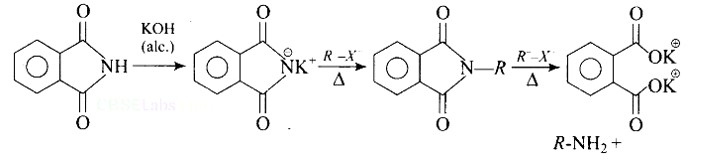
Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis produces primary amine. This process is known as Gabriel phthalimide reaction. The number of carbon atoms in the chain of amines of product is same as reactant.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is c.
In the cyclic structure of glucose or fructose, anomeric carbon is carbon that is close to an oxygen atom. As shown in the structure above, atoms 'a' and 'b' are close to the oxygen atom, and the hydroxyl groups of both carbon atoms have different orientations.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Among primary, secondary, and tertiary amines, tertiary amines are the most volatile compounds as they don't have any strong intermolecular H-bonding between N-H like the primary and secondary amines have.
So due to weak dipole-dipole interactions, (B) will be the most volatile.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
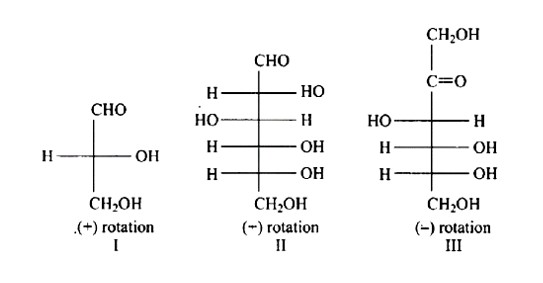
The Correct option is a.
In the same way as (+) glyceraldehyde has a group on the lowest asymmetric carbon on the right side, the I, II, and III structures have a (-OH) group on the lowest asymmetric carbon on the right side giving them a D-configuration.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers