Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Fill in the Blanks Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Hydrogen iodide is a more powerful reducing agent than sulphuric acid, it reduces the amount of iodine in the solution from H2SO4 to SO2 and HI to I2
When chloride salts are treated with sulfuric acid, HCl gas is formed, which produces a colourless gas.
NaCl + H2SO4→HCl + Na2SO4
Violet fumes are produced during the reaction due to the creation of iodine (I2) gas.
2NaI + H2SO4→Na2SO4 + 2HI ![]() 2H2O + SO2 + I2
2H2O + SO2 + I2
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The following reaction with silver nitrate demonstrates phosphinic acid reducing behaviour:
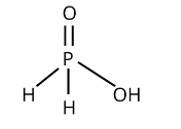
4AgNO3 + 2H2O + H3PO2→4Ag + 4HNO3 + H3PO4
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
PCl5 + 2Ag→2AgCl + PCl3
AgCl + 2NH3 (aq)→ [Ag (NH3)2] + Cl-
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
When nitric acid is mixed with copper metal, it produces distinct oxidation products.
3Cu + 8HNO3 (dil.)→3Cu (NO3)2 + 2NO + 4H2O
Cu + 4HNO3 (Conc)→3Cu (NO3)2 + 2NO2 + 2H2O
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
