Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
The C-atom in the CO32- ion undergoes sp2 hybridization. BF4, NH4+ and SO42- have a tetrahedral structure, whereas it has a triangular planar structure.
New answer posted
7 months agoContributor-Level 10
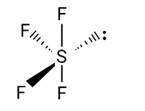
This is a Long Answer Type Question as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Na (H2)PO2
1+ (2x+1)+ x +2 (−2)=0
x−1=0
x=1
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
The catalytic oxidation of ammonia produces NO gas, which is used to make HNO3. 4 moles of NH3 created 4 moles of NO in the equation below. As a result, the moles of NO produced by oxidising two moles of NH3 will be two moles.
4NH3 + 5O2→4NO + 6H2O.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Sol:
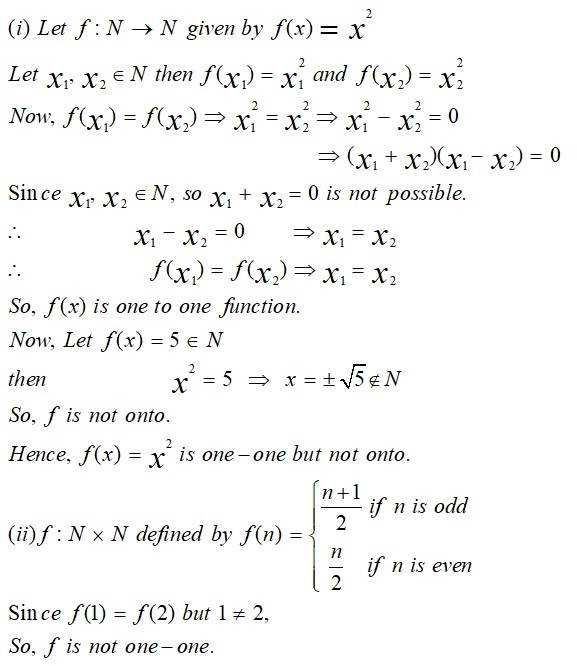
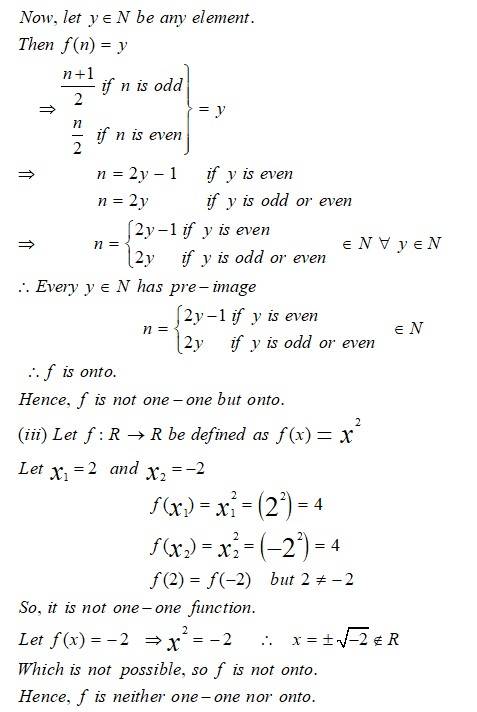
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
We get N2 in both situations when we heat ammonium dichromate and barium azide separately.
(NH4)2Cr2O7→Cr2O3 + 4H2O + N2
Ba (N3)2→Ba + 3N2.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii)
Bismuth is the sole element that has an inert pair effect. Only generates trihalides and has a +3 oxidation state. Florine, on the other hand, is small and has a high electronegativity.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Sol:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
