Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
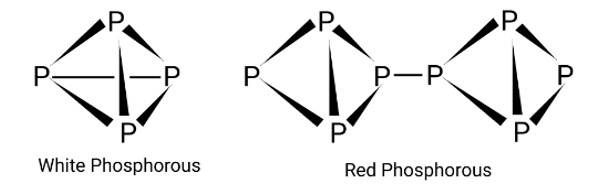
The crystal structure of red phosphorus features a sophisticated network of bonding, whereas white phosphorus is made up of P4 molecules. To avoid natural combustion, white phosphorus must be stored in water, but red phosphorus is stable in air.
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
On interaction with P4O10 forms an oxide of nitrogen, N2O5, and metaphosphoric acid, 3, nitric acid is formed. HPO3
HNO3 + P4O10→4HPO3 + 2N2O5
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Three oxoacids are
(a) HNO2, Nitrous acid
(b) HNO3, Nitric acid
(c) Hyponitrous acid, H2N2O2
3HNO2![]() + H2O + 2NO
+ H2O + 2NO
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
P4+6Cl2→4PCl3… (i)
PCl3+3H2O→H3PO3+3HCl *4
On adding eq. (i) and (ii)
P4+6Cl2+12H2O→4H3PO3+12HCl
1 mol of white phosphorus produces 12 mol of HCl
62 g of white phosphorus has been taken which is equivalent to = mol. Therefore 6 molHCl will be formed.
Mass of 6 molHCl=6*36.5=219.0g HCl
New answer posted
7 months agoContributor-Level 10
This is a True or False Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
P4O6 + 6H2O→4H3PO3 (i)
Next is neutralization
4 times H3PO3 + 2NaOH→Na2HPO3 + 2H2O (ii)
On addition of 2 equations
P4O6+8NaOH→4Na2HPO3+2H2O
P4O6 ( mol.mass )= (4*31+16*6)=220
Number of moles of P4O6 = =
The product formed is neutralized 8 moles of NaOH
P4O6 = 8* = molNaOH
Molarity of NaOH in 1 litre = 0.1M
Molarity =
Volume =
= * = 0.4 L
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
