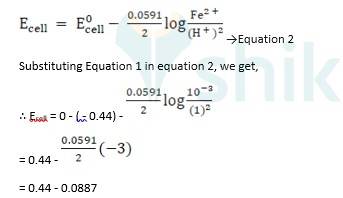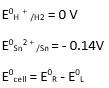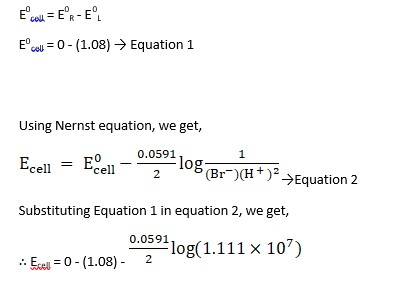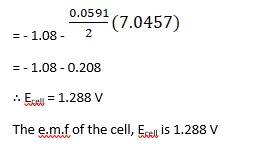Electrochemistry
Get insights from 145 questions on Electrochemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Electrochemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
Given -
Molarity, C = 0.00241 M
Conductivity, κ = 7.896 * 10–5 S cm–1
Molar conductivity? m =?
? 0m for acetic acid = 390.5 S cm2mol–1
Molar conductivity? m = k/c X 1000 S cm2 mol-1
= 7.896 X 10–5 S cm–1X 1000 cm3 L-1 / 0.00241 mol L-1
∴? m = 32.76 S cm2 mol-1
To calculate the dissociation constant, Ka, we use
Ka = → Equation 1
Here, we need to find the value of α (degree of dissociation), by the formula,
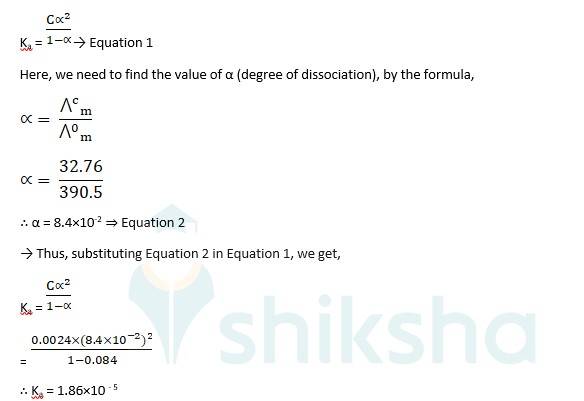
The molar conductivity? m is 32.76 S cm2 mol-1 and the dissociation constant, Ka is 1.86*10-5
New answer posted
9 months agoContributor-Level 10
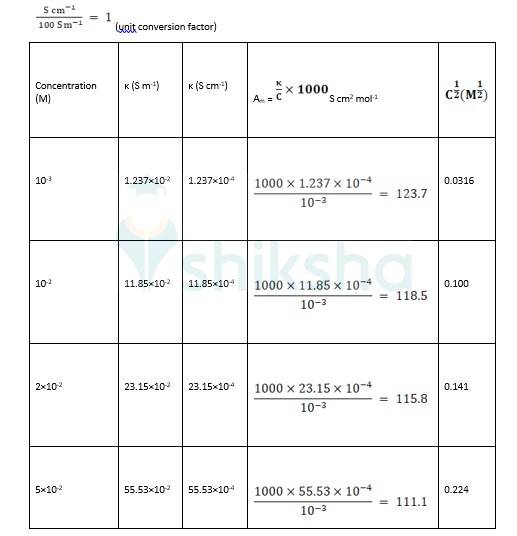
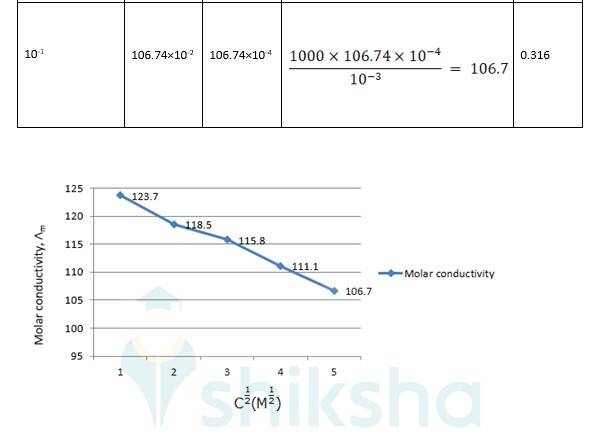
? 0 R = Intercept on the? axis = 124.0 S cm2 mol-1, which is obtained by extrapolation to zero concentration.
New answer posted
9 months agoContributor-Level 10
Given -
Resistance of a conductivity cell, R = 1500 Ω
Electrolytic conductivity of a solution, κ = 0.146 * 10-3 S cm-1
Cell constant =?
Conductivity, κ = cell constant/resistance
Cell constant = κ * R
= 0.146 * 10-3 S cm-1*1500 Ω
Cell constant = 0.219 cm-1
The cell constant of the cell containing 0.001M KCl solution at 298 K is 0.219 cm-1
New answer posted
9 months agoContributor-Level 10
Given -
Molarity, C = 0.20 M
Electrolytic conductivity of a solution, κ = 0.0248 S cm-1
Molar conductivity =?
Molar conductivity, ∧m = K/C X 1000 S cm2 mol-1
=0.0248S cm-1 X 1000 Cm3L-1 / 0.20 mol L-1
∴ ∧m = 124 S cm2 mol-1
Molar conductivity (∧m) of 0.20 M solution of KCl at 298 K is 124 S cm2 mol-1
New answer posted
9 months agoContributor-Level 10
The conductivity of a solution is defined as the conductance of one unit volume of solution kept between two platinum electrodes with a unit area of cross-section and at a distance of unit length.The molar conductivity of the solution is defined as the conducting power of all the ions produced by
one gram mole of an electrolyte in a solution. It is denoted by ∧m.
The conductivity of a solution (both for strong and weak electrolytes) always decreases with the decrease in concentration of the electrolyte i.e., on dilution. This pattern is seen because the number of ions per unit volume that carry the current in the solution d
New answer posted
9 months agoContributor-Level 10
Given - Zn → Zn2+ + 2e -, E0 = 0.76V (anode)
Ag2O + H2O + 2e - →2Ag + 2OH -, E0 = 0.344V (cathode), n = 2
ΔrG0 =?
Ecell0=?
Zn is oxidized and Ag2O is reduced.
Hence, the standard cell potential, Ecell0 is given as,
Ecell0 = ER0 - EL0
E0 cell = 0.344 + 0.76
∴ E0cell = 1.104 V
To calculate the standard Gibb's free energy? rG0, we use,
? rG0 = - nE0F → Equation 1
= - 2*96487*1.104 J
= - 213043.296 J
∴? rG0 = - 2.13*105 J
The standard cell potential, E0cell is 1.104 V and the standard Gibb's free energy? rG0 is - 2.13*105 J
New answer posted
9 months agoContributor-Level 10
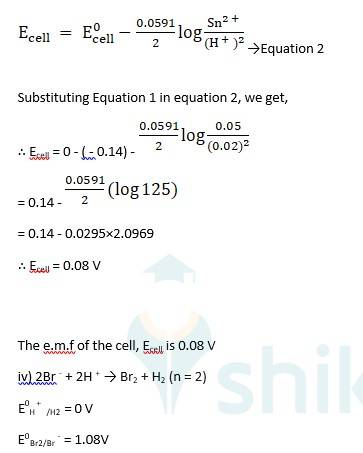
(i) Mg(s)|Mg 2+(0.001M)Cu2+(0.0001 M)|Cu(s) (ii) Fe(s)|Fe 2+(0.001M)H+ (1M)|H2 (g)(1bar)| Pt(s) (iii) Sn(s)|Sn2+(0.050 M)H+ (0.020 M)|H2 (g) (1 bar)|Pt(s) (iv) Pt(s)|Br – (0.010 M)|Br2 (l )H+ (0.030 M)| H2 (g) (1 bar)|Pt(s).
A 3.5 Ecell = ?
(i) Mg + Cu2+ → Mg2+ + Cu (n = 2)
E0 Cu2+ / Cu+ = 0.34V
E0 Mg 2+ / Mg = - 2.37 V
Ecell0 = ER0-EL0
Ecell0 = 0.34 - ( - 2.37) → Equation 1
Using Nernst equation, we get,
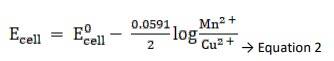
Substituting Equation 1 in equation 2, we get,
∴ Ecell = 0.34 - ( - 2.37) - 0.0591 / 2 log 10 -3/ 10-4
= 2.71 - 0.0591 / 2 log 10
= 2.71 - 0.02955
∴ Ecell = 2.68 V
The e.m.f of the cell, Ecell is 2.68 V
ii) Fe + 2H + →
New answer posted
9 months agoContributor-Level 10
(1) Known - E0Cr3+/Cr = - 0.74 V
E0 cd2+ = - 0.40 V
? rG0 =? K =?
The galvanic cell of the given reaction is written as - Cr (s)|Cr3+ (aq)| Cd2+ (aq)|Cd (s)→ Reaction 1
Hence, the standard cell potential is given as, E0 = ER0 - EL0
= - 0.40 - (- 0.74)
∴ E0 = + 0.34 V
To calculate the standard Gibb's free energy? rG0, we use,
? rG0 = - nE0F → Equation 1
wherenF is the amount of charge passed and E0 is the standard reduction electrode potential. Substituting n = 6 (no. of e - involved in the reaction 1), F = 96487 C mol-1,
E0 = + 0.34 V in Equation 1, we get, l
? rG0 = - 6*0.34V*96487 C mol-1
= - 196833.48 CV mol-1
= - 196833.48 J mol-1
∴?
New answer posted
9 months agoContributor-Level 10
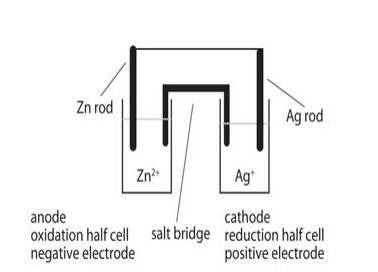
The galvanic cell corresponding to the given redox reaction can be represented as:
Zn|Zn2+ (aq)|Ag + (aq)|Ag
- 1) Zn electrode (anode) is negatively charged because, at this electrode, Zn is oxidized to Zn2+, causing electron accumulation at the
- 2) Electrons (ions) are the carriers of the current in the cell and in the external circuit, current flows from Ag (cathode) to Zn (anode) which is normally opposite to the electron flow which is from anode to cathode.
- 3) At anode:
Zn (s)⇒ Zn2 + (aq) + 2e– At cathode:
Ag + (aq) + e –⇒ Ag (s)
New answer posted
9 months agoContributor-Level 10
K + /K = –2.93V, Ag+ /Ag = 0.80V, Hg2+/Hg = 0.79V Mg2+/Mg = –2.37 V, Cr3+/Cr = – 0.74V
A 3.2 Reducing power of metals increase with the decrease of reduction potential. Hence, the increasing order of reducing power will be as,
Ag < Hg < Cr < Mg < K
When the reduction potential is lower, the element has more tendency to get oxidized and thus more will be reducing power. The metal that has more negative electrode potential will be the one with more reducing power. Thus, here potassium (K) has the highest reducing power among the given elements.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers