Electrochemistry
Get insights from 145 questions on Electrochemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Electrochemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
The order in which the given metals displace each other from the solution of their salts is given by,
Mg>Al> Zn> Fe> Cu
A metal of stronger reducing power displaces another metal of weaker reducing power from its solution of salt. The order of increasing the reducing power of given metals is Cu< Fe< Zn< Algiven metals displace each other from the solution of their salts is given by, Mg>Al> Zn> Fe> Cu. This is hence arranged in decreasing order of its reactivity
New answer posted
9 months agoContributor-Level 10
In the corrosion reaction, due to the presence of air and moisture, oxidation takes place at a particular point of an object made of iron. That spot behaves as the anode. The reaction at the anode is given by,
Fe (s) ⇒ Fe2+ (aq) + 2e-
Electrons released at the anodic spot move through the metal and go to another spot of the object, wherein presence of H+ ions, the electrons reduce oxygen. This spot behaves as the cathode. These H+ ions come either from H2CO3, which are formed due to the dissolution of carbon dioxide from the air into water. The cathodic reaction is given by
O2 (air) + 4Haq++4e-⇒ 2H O
The overall reaction is given by,
New answer posted
9 months agoContributor-Level 10
Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
New answer posted
9 months agoContributor-Level 10
Anode: Lead (Pb)
Cathode: a grid of lead packed with lead oxide (PbO2)
Electrolyte: 38% solution of sulphuric acid (H2SO4)
The cell reactions are as follows :
Pb (s) + SO2-4 (aq) ⇒ PbSO4 (s) + 2e- (anode)
PbO2 (s) + SO2-4 (aq) + 4H+ (aq) +2e-⇒ PbSO4 (s) +2H2O (l) (cathode)
Pb (s) + PbO2 (s) +2H2SO4 (aq)⇒ 2PbSO4 (s) +2H2O (l)
(overall cell reaction)
On charging, all these reactions will be reversed.
New answer posted
9 months agoContributor-Level 10
Cr2O72– + 14H+ + 6e–⇒ 2Cr3+ + 7H2O
A 3.12
Cr2O72– + 14H+ + 6e–⇒ 2Cr3+ + 7H2O
For reducing one mole of Cr2O72–, 6 mole of electrons are required. Hence, 6 Faraday charges is needed. Hence, 6F = 6*96487 = 578922 C. Thus, the quantity of electricity is needed is 578922 C.
New answer posted
9 months agoContributor-Level 10
Metals with greater reactivity can be extracted electrolytically. Sodium, potassium, calcium, lithium, magnesium, aluminium which are present in the top of the reactivity series are extracted electrolytically.
New answer posted
9 months agoContributor-Level 10
Current I = 0.5A
Time t = 2hrs = 2*60*60 = 7200 seconds
Charge Q = I * t
Q = 0.5*7200 = 3600 C
Charge carried by 1 mole of electrons (6.023*1023electrons) is equal to 96487C.
No of electrons = 6.023*1023 * 3600/96487
No of electrons = 2.25*1022 electrons
New answer posted
9 months agoContributor-Level 10
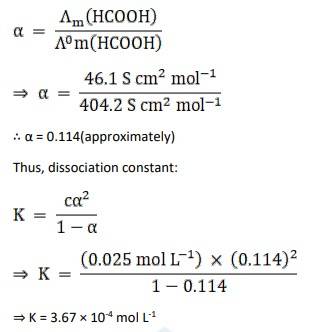
C = 0.025 mol L-1
Am = 46.1 Scm2 mol L-1
λ0 (H+) = 349.6 Scm2 mol L-1
λ0 (HCOO-) = 54.6 Scm2 mol L-1
Λ0m (HCOOH) = Λ0 (H+) + Λ0 (HCOO-)
= 349.6 + 54.6
= 404.2 S cm2 mol L-1
Now, the degree of dissociation:
New question posted
9 months agoNew answer posted
9 months agoContributor-Level 10
The conductivity of a solution depends on the amount of ions present per volume of the solution. When diluted, the concentration of the ions decreases which implies that the number of ions per volume decreases thus, in turn, conductivity decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
