Electrochemistry
Get insights from 145 questions on Electrochemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Electrochemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
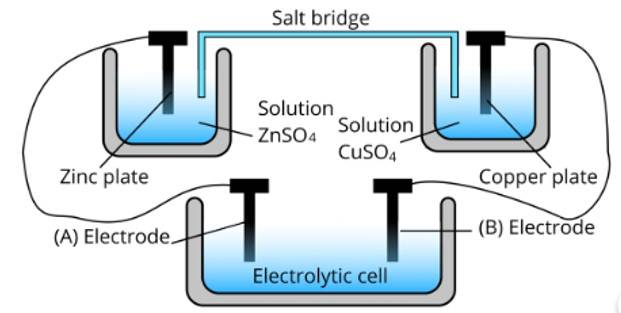
Ans: (i) Cell 'B' will act as an electrolytic cell because to less value of Ecell The reactions occurring in the cell are as follows;
At anode: Zn2+ + 2e− → Zn
At cathode: Cu (s) → Cu2+ + 2e−
(ii) Cell 'B' has a higher emf so it acts as a galvanic cell. The reactions are as follows;
At anode: Zn → Zn2+ + 2e−
At cathode: Cu2+ + 2e− → Cu
New question posted
8 months agoNew answer posted
9 months agoContributor-Level 10
Given -
(i) All the ions are in aqueous state.
Reaction in solution:
AgNO3 (s) + aq → Ag + + NO3-
H2O → H + + OH -
At cathode:
Ag + (aq) + e - →Ag (s)
Ag + ions have lower discharge potential than H + ions. Hence, Ag + ions get deposited as Ag in preference to H + ions.
At anode:
Ag (s)→ Ag + (aq) + e -
As Ag anode is attacked by NO3- ions, Ag of the anode will dissolve to form Ag + ions in the aqueous solution.
(ii) Reaction in solution:
AgNO3 (s) + aq → Ag + + NO3-
H2O óH + + OH -
At cathode:
2Ag + (aq) + 2e - →2Ag (s)
Ag + ions have lower discharge potential than H + ions. Hence, Ag + ions get deposited as Ag in preference
New answer posted
9 months agoContributor-Level 10
The electrode reaction is written as,
2Fe3+ + 2I - → 2Fe2+ + I2
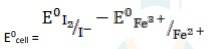
= 0.54V - 0.77V
∴ E0cell = - 0.23 V
It is not feasible, as E0cell is negative, ∴ ?G0 is positive.
- The electrode reaction is written as,
- 2Ag+ (aq) + Cu(s)→ Cu2+ (aq) + Ag(s)
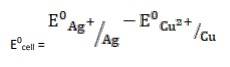
= + 0.80V - 0.34V
∴ E0cell = 0.46V
It is feasible, as Ecell 0 is positive, ∴ ?G0 is negative.
- (iii) The electrode reaction is written as,
- 2Fe3+ (aq) + 2Br- (aq)→ 2Fe2+ (aq) + Br2
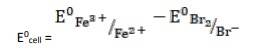 k
k= 0.77V - 1.09V
∴ E0cell = - 0.32 V
It is not feasible, as E0cell is negative, ∴ ?G0 is positive.
- (iv) The electrode reaction is written as,
- Ag(s) + Fe3+ (aq) → Fe2+ (aq) + Ag+ (aq)
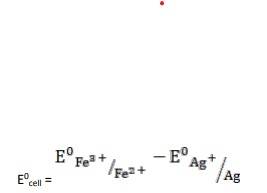
= 0.77V - 0.80V
∴ E0cell = -
New answer posted
9 months agoContributor-Level 10
Equivalent weight is Ag, EAg = 180/1 = 180
Equivalent weight is Cu, ECu = 63.5 / 2 = 31.75
Equivalent weight is Zn, EZn= 65/2 = 32.5
Using Faraday's second law of electrolysis, to find the mass of Cu and Zn, we use Equation 1,
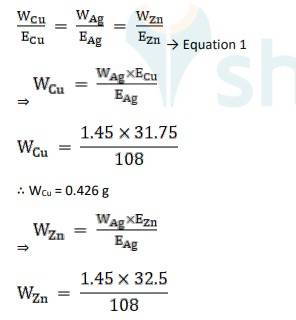
∴ WZn = 0.436 g
To find the time of current flow, using Faraday's first law of electrolysis we get,
M = Z *I *t ⇒ Equation 2
? Z = Equivalent Weight / 96487, Equation 2 becomes,
M = 108 / 96487 X 1.5 X t
t = 1.45 X 96487 / 108X 1.5
t = 864 seconds.
The time of current flow, t = 864 seconds, the mass of Cu is 0.426 g and mass of Zn is 0.436 g
New answer posted
9 months agoContributor-Level 10
Quantity of electricity passed = 5 A * (20 * 60 sec)
= 6000 C ⇒ Equation 1
The electrode reaction is written as,
Ni2+ + 2e → Ni
Thus, the quantity of electricity required = 2F
= 2*96487 C
= 192974 C
? 192974 C of electricity deposits 1 mole of Ni, which is 58.7 g ⇒ Equation 2
Thus, equating equations 1 and 2, we get
192974 C of electricity deposits = 58.7 g
6000 C of electricity will deposit = 58.7 X 6000 / 192974
= 1.825g of Ni
The mass of Ni deposited at the cathode is 1.825g of Ni
New answer posted
9 months agoContributor-Level 10
(i) The electrode reaction for 1 mole of H2O is given as,
H2O → H2 + 1/2O2
i.e., O2- →1/2 O2 + 2e -
∴ The quantity of electricity required = 2F
= 2*96487 C
= 192974 C
The quantity of electricity required in coulomb for the oxidation of 1 mol of H2O to O2 is 192974 C
(ii) The electrode reaction for 1 mole of FeO is
FeO + 1/2 O2 → 1/2 Fe2O3
i.e., Fe2+ → Fe3+ + e -
∴ The quantity of electricity required = 1F
= 1*96487 C
= 96487 C
The quantity of electricity required in coulomb for the oxidation of 1 mol of FeO to Fe2O3 is 96487 C
New answer posted
9 months agoContributor-Level 10
(i) Ca2+ + 2e- → Ca
⇒ Here, 1 mole of Ca, i.e., 40g of Ca requires = 2 F electricity (F if Faraday)
∴ 20g of Ca requires = 20X2/40
= 1 F of electricity
Electricity in terms of Faraday required to produce 20.0 g of Ca from molten CaCl2 is 1 F of electricity.
(ii) Al3+ + 3e- → Al
⇒ 1 mole of Al, i.e., 27g of Al requires = 3 F electricity (F if Faraday)
∴ 40.0 g of Al will require = 3/27 X 40
= 4.44 F of electricity
Electricity in terms of Faraday required to produce 40.0 g of Al from molten Al2O3 is 4.44 F of electricit
New answer posted
9 months agoContributor-Level 10
The electrode reaction is given as,
Al3+ (aq) + 3e- → Al (s)
∴ The quantity of charge required for the reduction of 1 mol of Al3+ = 3F
= 3*96487 C
= 289461 C
The electrode reaction is given as,
Cu2+ (aq) + 2e- → Cu (s)
∴ The quantity of charge required for the reduction of 1 mol of Cu2+ = 2F
= 2*96487 C
= 192974 C
The electrode reaction is given as, MnO4→ Mn2+
i.e., Mn7+ + 5e - → Mn2+
∴ The quantity of charge required for the reduction of 1 mol of Mn7+ = 5F
= 5*96487 C
= 482435 C
New question posted
9 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers