Hydrocarbons
Get insights from 111 questions on Hydrocarbons, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrocarbons
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
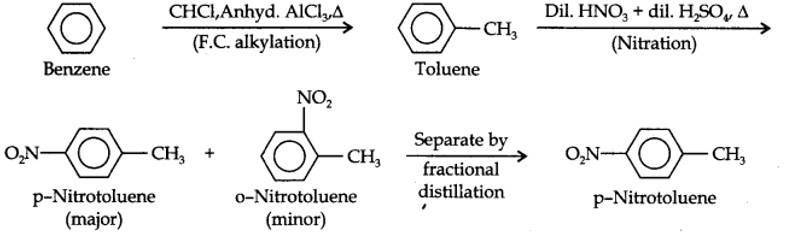
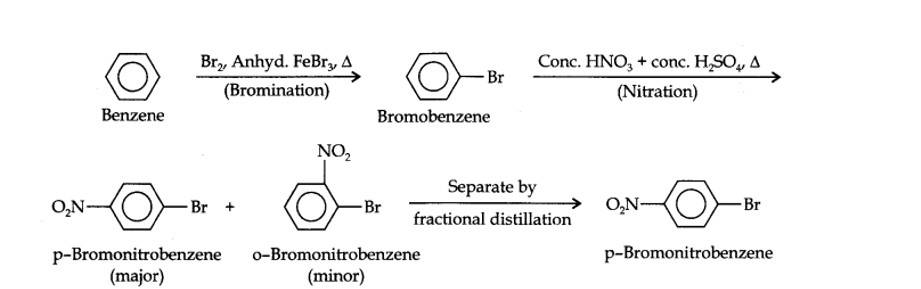
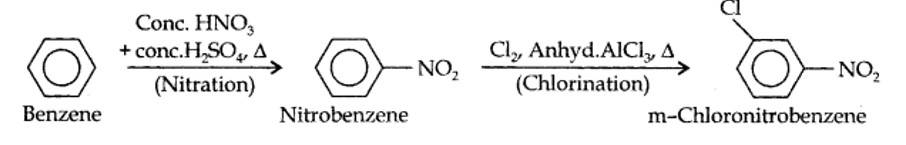
(i) The two substituents in the benzene ring are present at p-positions. Therefore, the sequence of reactions should be such that first an o, p-directing group, i.e., Br atom should be introduced in the benzene ring and this should be followed by nitration. Thus,
New answer posted
8 months agoContributor-Level 10
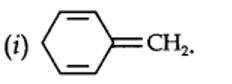
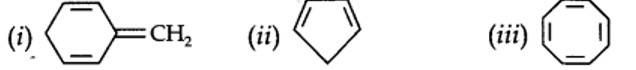
planar. It does contain six n-electrons but the system is not fully conjugated since all the six n-electrons do not form a single cyclic electron cloud which surrounds all the atoms of the ring. Therefore, it is not an aromatic compound.
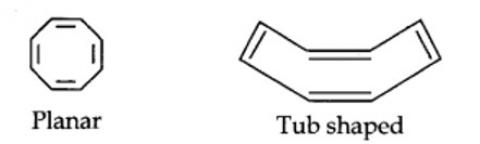
Cyclo-octatetraene is not planar but is tub shaped. It is, therefore, a non-planar system
New answer posted
8 months agoContributor-Level 10
The necessary conditions for a molecule to be aromatic are:
- It should have a single cyclic cloud of delocalised n-electrons above and below the plane of the molecule.
- It should be planar. This is because complete delocalization of n-electrons is possible only if the ring is planar to allow cyclic overlap of p-orbitals.
- It should contain Huckel number of electrons, i.e., (4n + 2) n-electrons where n = 0, 1, 2, 3 etc.
A molecule which does not satisfy any one or more of the above conditions is said to be non-aromatic.
New answer posted
8 months agoContributor-Level 10
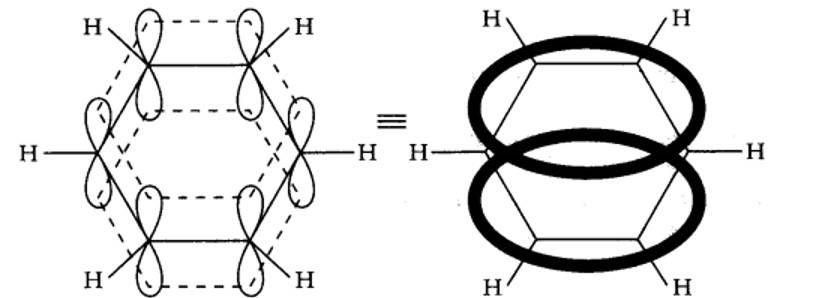
Benzene is a resonance hybrid of two canonical forms. In the resonance hybrid, all the six pi electrons are completely delocalized. This results in resonance stabilization.
New answer posted
8 months agoContributor-Level 10
The structures of cis- and trans-isomer of hex-2-ene are:
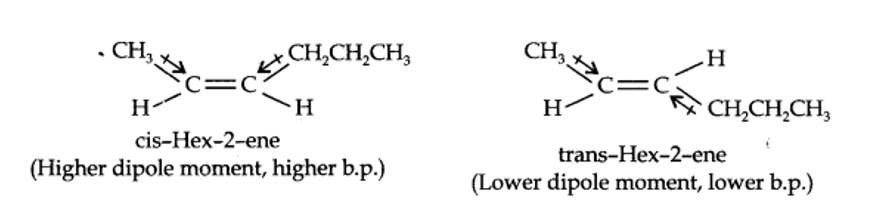
The boiling point of a molecule depends upon dipole-dipole interactions. Since cis-isomer has higher dipole moment, therefore, it has higher boiling point.
New answer posted
8 months agoContributor-Level 10
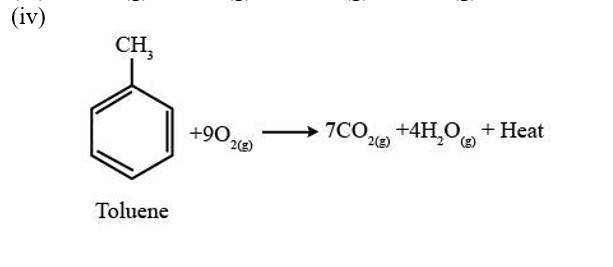
A combustion reaction is a reaction in which a substance reacts with oxygen gas, there is a formation of carbon dioxide, water with the evolution of light and heat.
(i) 2C4H10 (g) +13 O2 (g)?8CO2 (g)+10H2O (g) + Heat
(ii) 2C5H10 (g) +15 O2 (g)?10CO2 (g)+10H2O (g) + Heat
(iii) 2C6H10 (g) +17 O2 (g)?12CO2 (g)+10H2O (g) + Heat
New answer posted
8 months agoContributor-Level 10
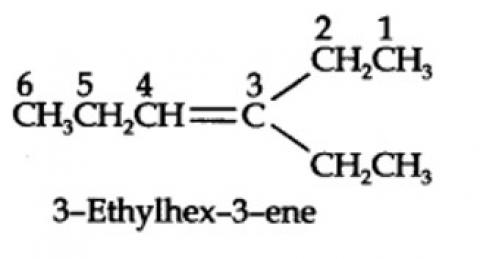
The ozonolysis of 4-Ethylhex-3-ene gives propanal and pentan-3-one.
The structural formula of the alkene (4-Ethylhex-3-ene) is as shown.
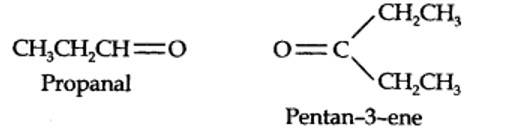
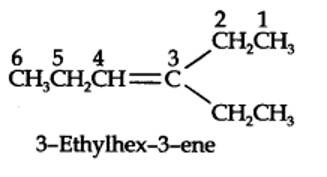
New answer posted
8 months agoContributor-Level 10
(i) An aldehyde with molar mass of 44 u is ethanal, CH3CH=0
(ii) Write two moles of ethanal side by side with their oxygen atoms pointing towards each other.
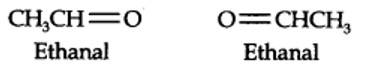
New answer posted
8 months agoContributor-Level 10
Step 1. Write the structure of the products side by side with their oxygen atoms pointing towards each other.
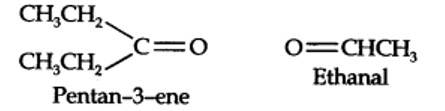
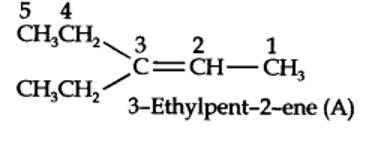
New question posted
8 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers