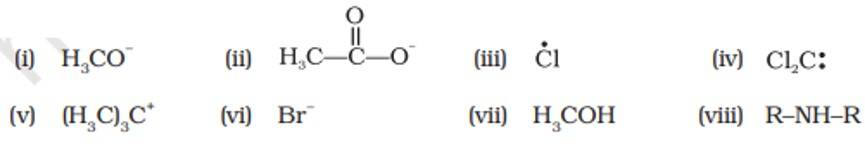Hydrocarbons
Get insights from 111 questions on Hydrocarbons, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrocarbons
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iv) C > B > D > A
The boiling point increases with the increase in the length of the side chain. However, with the branching, the boiling point decreases due to the decrease in the surface area.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
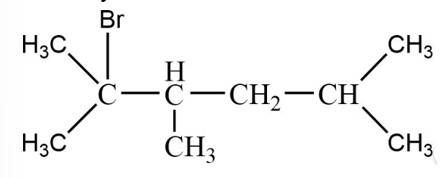
The reaction is
The alkane is
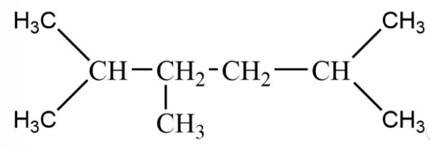
The tertiary bromide is
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The 2-methylpropane would lead to two types of radicals.
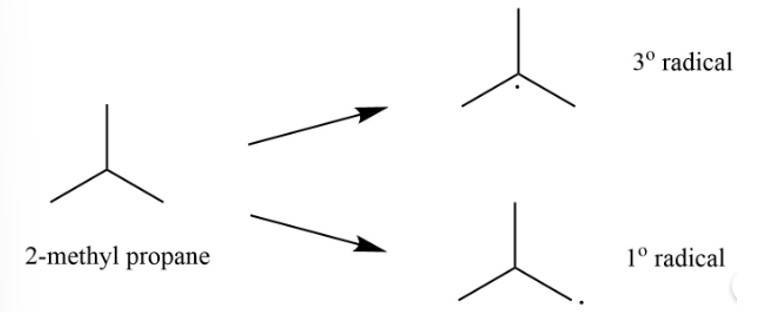
Among the two 3o radical is most stable as it contains 9 ∝ -hydrogen whereas 1o radical contains only 1 ∝ -hydrogen.
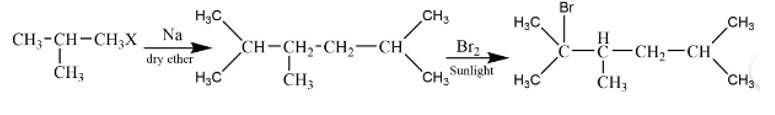
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
It is a Wurtz reaction where alkane is formed.
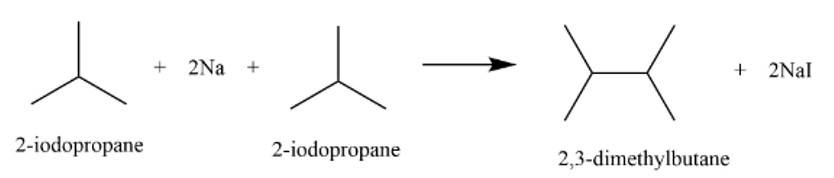
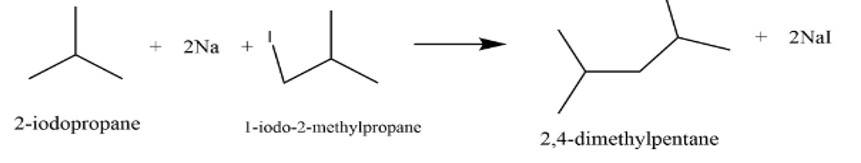
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The structure of 2-methylbutane is
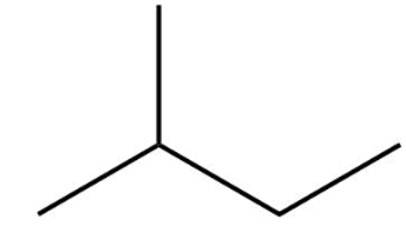
2-methylbutane
Total number | Total amount of monochlorinated Product | % of monochlorinated product | |
1° | 9 | 9 | 41.7 |
2° | 2 | 7.6 | 35.2 |
3° | 1 | 5 | 23.1 |
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Electrophiles | Nucleophiles |
(iii), (iv), (v) | (i), (ii), (vi), (vii), (viii) |
New answer posted
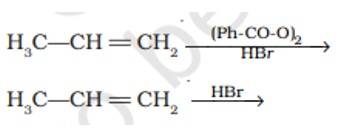
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
In presence of (Ph-CO-O)2it lead to CH3-CH2-CH2Br as the reaction undergo by free radical mechanism
However in absence of (Ph-CO-O)2 the reaction undergo by carbocation intermediate thus lead to give CH3-CHBr-CH3
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
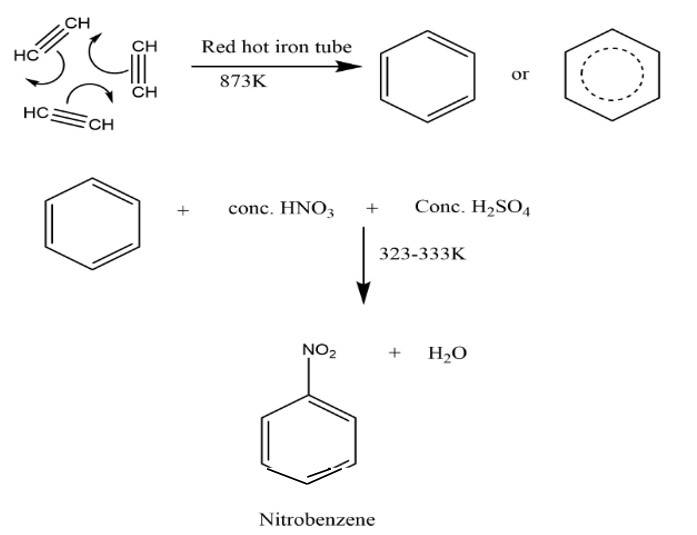
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The nitro group strongly deactivate the benzene towards the electrophilic substitution reaction due to the -R and -I effect
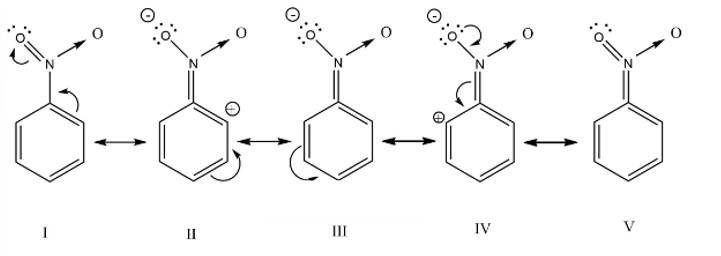
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Due to the +R effect halogens are ortho and para directing groups.
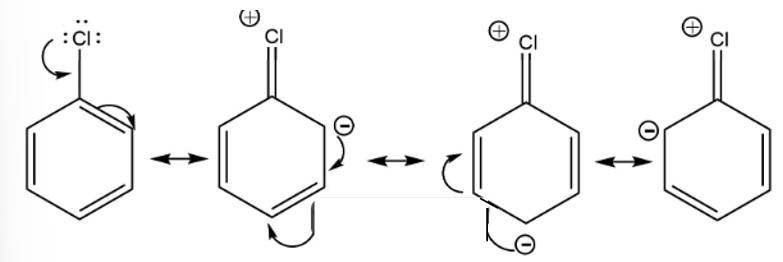
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers