Hydrocarbons
Get insights from 111 questions on Hydrocarbons, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrocarbons
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
The molar mass of the compound = 3.38 x 22400/896 =831gmol-1
Element | Percentage | At.mass | Relative ratio | the simplest ratio |
C | 87.8% | 12 | 7.31 | 3 |
H | 12.19% | 1 | 12.19 | 5 |
Thus the empirical formula of the compound is C3H5
Molecular formula =n x empirical formula = 831/41 x C3H5 = C6H10
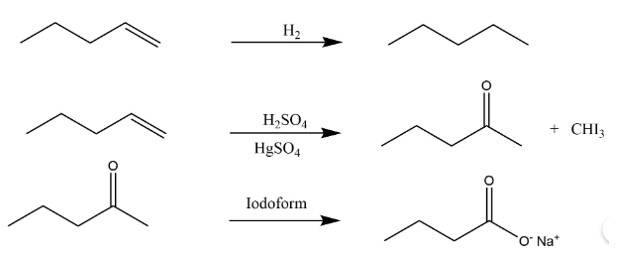
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
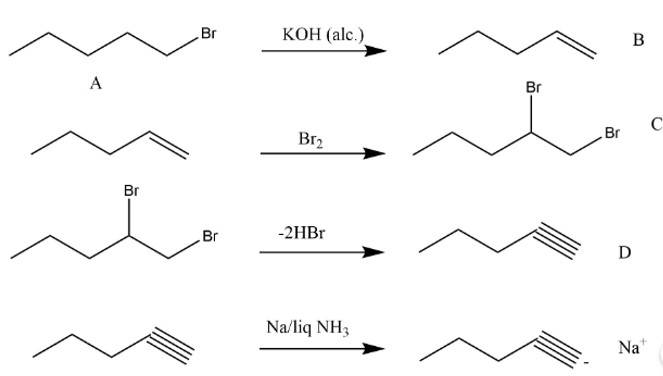
New answer posted
8 months agoContributor-Level 10
(a) Eutrophication: When the growth of algae increases in the surface of water, dissolved oxygen in water is reduced. This phenomenon is known as eutrophication. (Due to this growth of fish gets inhibited). (b) Pneumoconiosis: It is a disease which irritates lungs. It causes scarring or fibrosis of the lung. (c) Photochemical smog: Photochemical smog is formed as a result of photochemical decomposition of nitrogen dioxide and chemical reactions involving hydrocarbons. It takes place during dry warm season in presence of sunlight. It is oxidising in nature. (d) Classical smog: Classical smog is formed due to condensation of SO, vapours on particles of carbon in cold climate. It is generally formed during winter when there is severe cold.It is reducing in nature. |
New answer posted
8 months agoContributor-Level 10
Toxic substance that is used to kill insects are called insecticides. For example: DDT, BHC.
New answer posted
8 months agoContributor-Level 10
Carbon monoxide (CO), sulphur dioxide (SO2) and oxides of nitrogen (NO2).
New answer posted
8 months agoNew answer posted
8 months agoContributor-Level 10
Green chemistry can be applied as mentioned in the following examples:
(i) In dry-cleaning, use of liquefied CO2 in place of tetrachloroethene.
(ii) In bleaching of paper using hydrogen peroxide instead of chlorine.
(iii) In the manufacture of chemicals like ethanal using environment-friendly chemicals and conditions.
New answer posted
8 months agoContributor-Level 10
PCBs are polychlorinated biphenyls. They are contaminants of water. They are used as fluids in transformers and capacitors
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
