Hydrocarbons
Get insights from 111 questions on Hydrocarbons, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrocarbons
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iii) & (iv) Methane is a controlled oxidation reaction that leads to giving formaldehyde and methanol.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iii) As the unburn carbon left out in this reaction thus it is an incomplete reaction
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iv) The order of reactivity of β– elimination reaction is 3o>2o>1o
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) The electronegativity of sp hybridization is higher than that of sp3 hybridization.
The +I effect destabilizes the carbanion.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iii) The addition of alkyl halides to propene depends upon the H-X bond strength i.e. lesser the bond strength higher will be the reactivity of the alkyl halide. As down the group size of the halogens increases thus the bond strength decreases.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
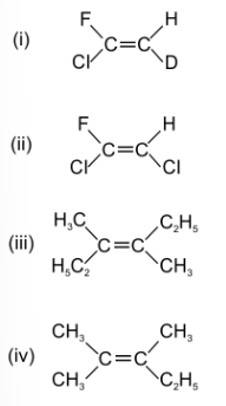
(iv) The two substituents attached to the same C atom of the C=C bond should be different to exhibit geometrical isomerisms.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
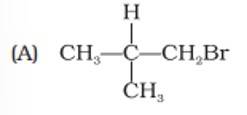
(i) The reaction follows Markovnikov's rule leading to giving 2o carbocation as an intermediate.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
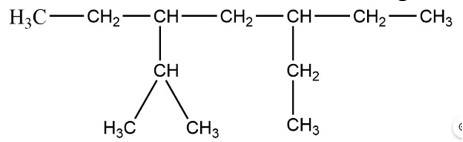
(i) 3, 6 – Diethyl – 2 – methyl octane
Octane is the longest chain here and the side chain follows the lowest sum rule.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
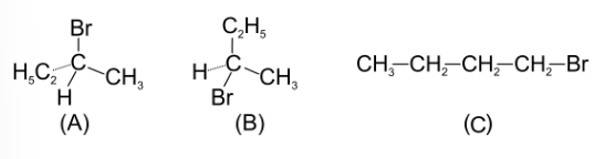
(ii) R–Cl< RBr < RI
The reduction of alkyl halides depends upon the C-X bond strength i.e. lesser the bond strength higher will be the reactivity of alkyl halide. As down the group size of the halogens increases thus the bond strength decreases.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) I2< Br2< Cl2< F2
The reactivity of halogens with alkanes depends on the electronegativity i.e. higher the electronegativity higher would be the reaction. As down the group reactivity decrease so thus its reactivity.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers