Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
(Natural abundance of 1H x molar mass ) + (Natural abundance of 2H x molar mass of 2H)
Natural abundance of 1H = 99.985
Natural abundance of 2H = 0.015
Average atomic mass =
= 1.00015u
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
sum of the number of protons and neutrons is same but the number of protons is different
Isobars are the atoms which have same mass number (sum of number of neutrons and protons) but different atomic number (i.e. different proton number)
For example, 146C and 147N.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
Overall neutrality of atom.
According to the J.J Thomson model the positive charge is uniformly distributed and the electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement just as watermelon of positive charge with plums or seeds (electrons) embedded into it. Thus this model is able to explain the overall neutrality of the atom
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
(a) Is this statement true?
Ans: Yes the given statement is true
(b) If yes, according to which law?
Ans: Multiple law of proportions: According to the Law of Multiple proportions, when two elements combine to generate more than one compound, the weights of one element that combine with a fixed weight of the other are in a ratio of tiny whole numbers.
(c) Give one example related to this law
Ans:
C (g) + O (g) -> CO (g)
12 g 16 g 28 g
C (g) + O2 (g) -> CO2 (g)
12 g 32 g &n
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
The mass of an electron is equal to the mass of a neutron.
The neutron is heavier than the electron as
Mass of the neutron = 1.67 x 10-27 kg
Mass of the electron = 9.11 x 10-31kg
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
If all gases are at the same temperature and pressure, Gay lussac's law of gaseous volumes states that gases combine or are created in a chemical reaction in a simple volume ratio.
H2 (g) + Cl2 (g) → 2HCL (g)
1 volume 1 volume 2 volume
22.4 litre 22.4 litre 44.8 litre
2N2 (g) + O2
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
Characteristics of cathode rays depend upon the nature of gas present in the cathode ray tube. As per the result obtained from the cathode ray discharge tube experiment the characteristics of cathode rays (electrons) does not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube. Which concludes that electrons are the basic constituent of all the atoms
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
ANS – Option (iv)
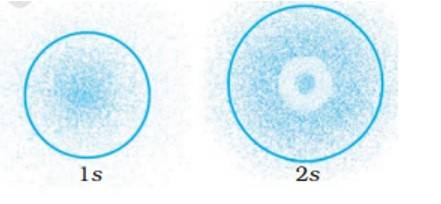
The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases. Electrons at the 1s orbital decreases as we move far from the nucleus, however in case of 2s the probability decreases initially then it increases with the distance and thereafter at a certain point it starts decreasing with the distance.
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Molecular mass of Ca3 (PO4)2 = 310.18 g / mole
Given mass of calcium 4 x 30= 120 g
Given mass of phosphorous = 31 x 2 =62 g
Given mass of oxygen = 16 x 8 = 128 g
Mass percent of Calcium = x 100 = 38.71%
Mass percent of Phosphorous = x 100 = 20%
Mass percent of Oxygen = x 100 = 41.29 %
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (ii) 1s2 2s2 2p6 3s2 3p6 3d9 4s2
As per the Hund's rule the half-filled and fully filled orbital leads to the extra stability due to the symmetry thus fully filled 3d and half-filled 4s is preferred.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers