Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
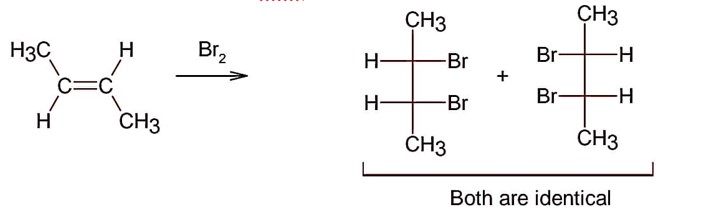
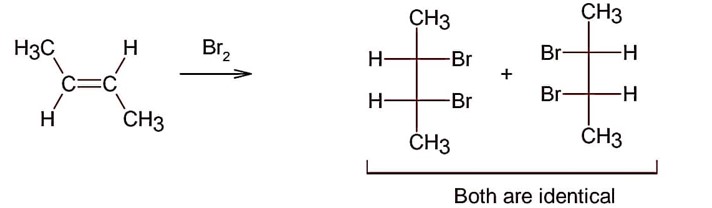
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New answer posted
3 months agoContributor-Level 10
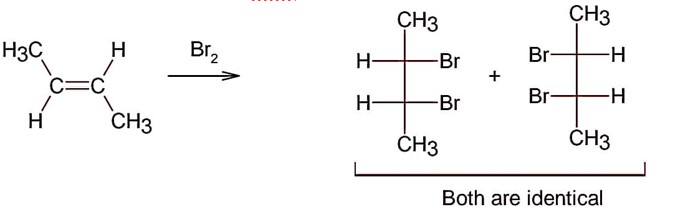
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New answer posted
3 months agoContributor-Level 10
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New answer posted
3 months agoContributor-Level 10
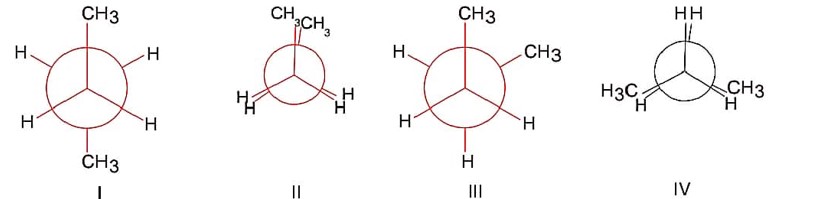
Structure (I) is anti conformer.
Structure (II) is fully eclipsed conformer.
Structure (III) is skew or gauche conformer.
Structure (IV) is partially eclipsed.
order of stability
(I) > (III) > (IV) > (II)
Order of P.E. is (II) > (IV) > (III) > (I).
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 9
2700 kJ energy released from 180 gm (1 mole) of glucose
1 kJ energy released from
10000 kJ energy released from
Amount of glucose =
New answer posted
3 months agoContributor-Level 10
Hydrogen peroxide reduces iodine to iodide ion is basic medium as;
New answer posted
3 months agoContributor-Level 10
w = 20 g
Mole of Na2O=
1 mole of Na2O gives 2 mole of NaOH
mole of Na2O gives moles of NaOH
Molarity of NaOH solution
= 1.29 M
=
Ans. = 13
New answer posted
3 months agoContributor-Level 9
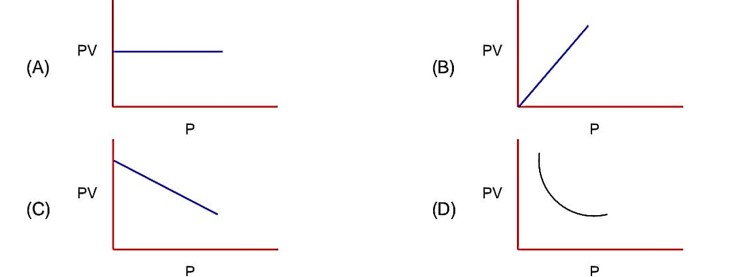
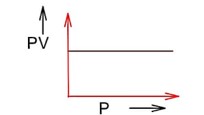
PV = nRT PV = constant (at constant T)
Pressure increases & volume decreases, PV remains constant at constant T.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers