Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
4 moles of HNO3 produced 3 mol of KNO3
Here mole of produced KNO3 =
If 3 mol of KNO3 produced by 4 moles of HNO3
1 mole of KNO3 produced by moles of HNO3
and mole of KNO3 produced by moles of HNO3 = 1.45 mole of HNO3
Hence mass of HNO3 = mole * mol.wt = 145 * 63 = 91.48 91.5gm
New answer posted
3 months agoContributor-Level 10
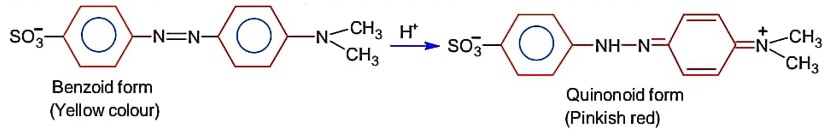
For an acid- base titration, Methly orange exist at end point as quinonoid form.
New question posted
3 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers