Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Aniline show acid-base reaction with AlCl3
aniline is a Lewis base while AlCl3 acts as lewis acid.
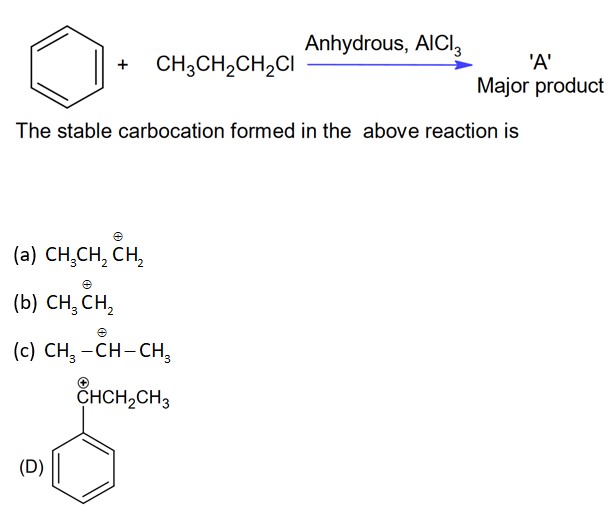
New answer posted
4 months agoContributor-Level 10
With AlCl3, alkyl halide will form cabocation which will show rearrangement.
New answer posted
4 months agoContributor-Level 10
In this carbocation +M effect of -OCH3 group stabilizes the carbocation.
While in option (A) and (B), +M of -OCH3 will not work but in option (C), +M of -OCH3 works so due to more delocalization in option (D), it is more stable.
New answer posted
4 months agoContributor-Level 10
Baking soda = NaHCO3
Washing soda = Na2CO3. 10H2O
Caustic soda = NaOH
New answer posted
4 months agoContributor-Level 10
From the given structure of CuSO4 . 5H2O Cu (II) ion and oxygen bonds are present but ligands coordinating with Cu (II) ion are not O and S both.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers