Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
New answer posted
6 months agoContributor-Level 10
Anode: Lead (Pb)
Cathode: a grid of lead packed with lead oxide (PbO2)
Electrolyte: 38% solution of sulphuric acid (H2SO4)
The cell reactions are as follows :
Pb (s) + SO2-4 (aq) ⇒ PbSO4 (s) + 2e- (anode)
PbO2 (s) + SO2-4 (aq) + 4H+ (aq) +2e-⇒ PbSO4 (s) +2H2O (l) (cathode)
Pb (s) + PbO2 (s) +2H2SO4 (aq)⇒ 2PbSO4 (s) +2H2O (l)
(overall cell reaction)
On charging, all these reactions will be reversed.
New answer posted
6 months agoContributor-Level 10
14.2
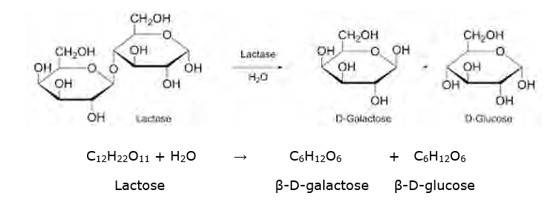
Lactose is a disaccharide carbohydrate (made up of two monosaccharide units) composed of β-D-galactose and β-D-glucose units. Hydrolysis breaks the glycosidic bond converting sucrose into β-D- galactose and β-D-glucose.
NOTE: But however, this reaction is so slow that it takes years for the solution of sucrose to undergo negligible change. Hence an enzyme called sucrase is added to proceed rapidly.
New answer posted
6 months agoContributor-Level 10
Cr2O72– + 14H+ + 6e–⇒ 2Cr3+ + 7H2O
A 3.12
Cr2O72– + 14H+ + 6e–⇒ 2Cr3+ + 7H2O
For reducing one mole of Cr2O72–, 6 mole of electrons are required. Hence, 6 Faraday charges is needed. Hence, 6F = 6*96487 = 578922 C. Thus, the quantity of electricity is needed is 578922 C.
New answer posted
6 months agoContributor-Level 10
Metals with greater reactivity can be extracted electrolytically. Sodium, potassium, calcium, lithium, magnesium, aluminium which are present in the top of the reactivity series are extracted electrolytically.
New answer posted
6 months agoContributor-Level 10
Current I = 0.5A
Time t = 2hrs = 2*60*60 = 7200 seconds
Charge Q = I * t
Q = 0.5*7200 = 3600 C
Charge carried by 1 mole of electrons (6.023*1023electrons) is equal to 96487C.
No of electrons = 6.023*1023 * 3600/96487
No of electrons = 2.25*1022 electrons
New answer posted
6 months agoContributor-Level 10
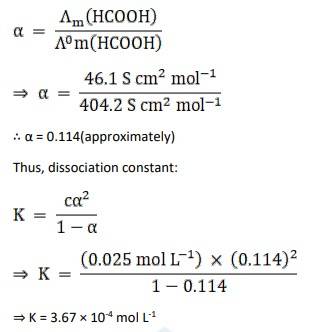
C = 0.025 mol L-1
Am = 46.1 Scm2 mol L-1
λ0 (H+) = 349.6 Scm2 mol L-1
λ0 (HCOO-) = 54.6 Scm2 mol L-1
Λ0m (HCOOH) = Λ0 (H+) + Λ0 (HCOO-)
= 349.6 + 54.6
= 404.2 S cm2 mol L-1
Now, the degree of dissociation:
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
The conductivity of a solution depends on the amount of ions present per volume of the solution. When diluted, the concentration of the ions decreases which implies that the number of ions per volume decreases thus, in turn, conductivity decreases.
New answer posted
6 months agoContributor-Level 10
Given:
2Fe3+ (aq) + 2I- (aq) → 2Fe2+ (aq) + I2 (s)
E0cell = 0.236V
n = moles of e- from balanced redox reaction = 2
F = Faraday's constant = 96,485 C/mol
T = 298 K.
Using the formula, we get
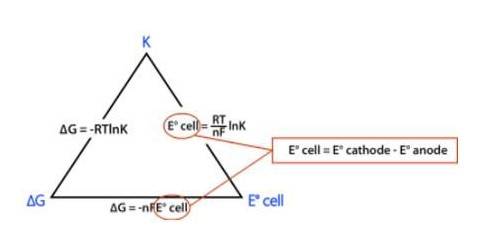
? rG0 = – nFE0cell
⇒? rG0 = – 2 * FE0cell
⇒? rG0 = −2 * 96485 C mol-1 * 0.236 V
⇒? rG0 = −45540 J mol-1
⇒? rG0 = −45.54 kJ mol-1
Now,
? rG0 = −2.303RT log Kc
Where, K is the equilibrium constant of the reaction
R is the gas constant; R = 8.314 J-mol-C-1
⇒ −45540 J mol-1 = –2.303* (8.314 J-mol-C-1)* (298 K) * (log Kc)
Solving for Kc we get,
⇒ logKc = 7.98
Taking antilog both side, we get
⇒ Kc = Antilog (7.98)
⇒ Kc = 9.6 * 1
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers