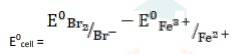Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
14.22
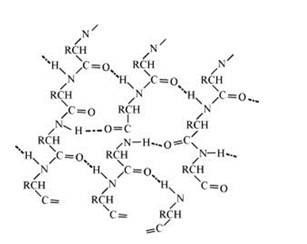
The stability of α-helix structure is due to intramolecular hydrogen bonding between –NH and –CO groups of polypeptide chain. The α-helix is known as 3.613 because each turn of α-helix contains approximately 3.6 amino acids and a 13-membered ring is formed by hydrogen bonding.
New answer posted
6 months agoContributor-Level 10
14.21
There are two common types of secondary structure of proteins:
(i) α–helix structure
(ii) β–pleated sheet structure
α–Helix structure
If the size of R-groups is quite large then the intramolecular bonds are formed between the C=O of one amino acid and the N-H group of the forth amino acid residue in the chain. This causes the polypeptide chain to coil up into a spiral structure called righ handed α-helix structure.
β -pleated sheet structure
In this conformation, the polypeptide chains lie side by side in a zig0zag manner with alternate R groups on the same side situated at fixed distances apart. The two such neighbouring pol
New answer posted
6 months agoContributor-Level 10
14.20
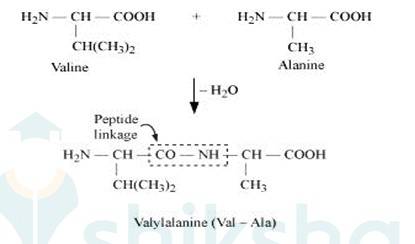
When two molecule of –amino acids similar or different combine in this way that the amino group of one molecule with carboxy group of the other molecule, this result in the elimination of water molecule and the formation of new bond-CO NH- .
This type of bond is called peptide bond or peptide linkage. The product of reaction is called dipeptide. For example, COOH group of valine combine with NH2 group of alanine, we get dipeptide Valylalanine (Val-Ala) as final product, as shown below:-
2- Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequ
New answer posted
6 months agoContributor-Level 10
14.19
(i) The amino acids which cannot be synthesised is called as essential amino acids. It must be obtained through diet. Example:- valine and leucine.
(ii) The amino acids which cannot be synthesised in the body is called as non-essential amino acids. Example: - glycine, Alanine.
New answer posted
6 months agoContributor-Level 10
14.18
Open structure of D-glucose could not explain the following reactions:
(i) Despite having the aldehyde group, glucose does not give 2,4 DNP test and Schiff's
(ii) Glucose does not react with sodium hydrogen sulphite to form addition
(iii) The absence of free –CHO group is shown when the penta-acetate of glucose does not react with hydroxyl
(iv) When glucose is heated with methanol in the presence of dry HCl gas, it forms two isomeric monomethyl derivatives known as? -D-glucoside and? -D-glucoside. Since only one molecule of methanol is used for the formation of methyl glucosided, these must be hemiacetals.
New answer posted
6 months agoContributor-Level 10
14.17
When D-glucose is heated and treated with HI for a long period of time, then n-hexane is formed, which shows that all the six-carbon atoms are linked in a straight
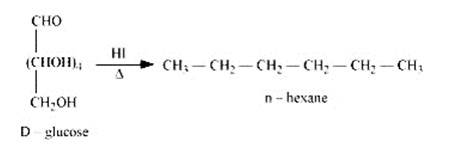
2. When D-glucose is treated with Br2 water i.e., bromine water which is a mild oxidising agent, then we get D-gluconic acid as one of the product. This reaction assures the presence of carbonyl group which is available as an aldehydic group.
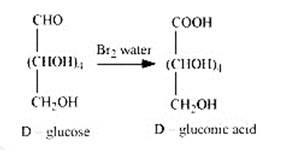
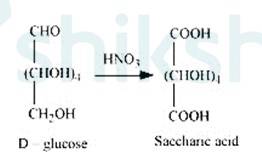
3. On being treated with HNO3, D-glucose get oxidised which gives saccharic acid as final Saccharic acid is a di-carboxylic acid. This reaction confirms the presence of alcoholic group (- OH) in the glucose.
New answer posted
6 months agoContributor-Level 10
Given -
(i) All the ions are in aqueous state.
Reaction in solution:
AgNO3 (s) + aq → Ag + + NO3-
H2O → H + + OH -
At cathode:
Ag + (aq) + e - →Ag (s)
Ag + ions have lower discharge potential than H + ions. Hence, Ag + ions get deposited as Ag in preference to H + ions.
At anode:
Ag (s)→ Ag + (aq) + e -
As Ag anode is attacked by NO3- ions, Ag of the anode will dissolve to form Ag + ions in the aqueous solution.
(ii) Reaction in solution:
AgNO3 (s) + aq → Ag + + NO3-
H2O óH + + OH -
At cathode:
2Ag + (aq) + 2e - →2Ag (s)
Ag + ions have lower discharge potential than H + ions. Hence, Ag + ions get deposited as Ag in preference
New answer posted
6 months agoContributor-Level 10
The electrode reaction is written as,
2Fe3+ + 2I - → 2Fe2+ + I2
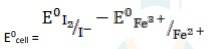
= 0.54V - 0.77V
∴ E0cell = - 0.23 V
It is not feasible, as E0cell is negative, ∴ ?G0 is positive.
- The electrode reaction is written as,
- 2Ag+ (aq) + Cu(s)→ Cu2+ (aq) + Ag(s)
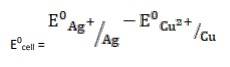
= + 0.80V - 0.34V
∴ E0cell = 0.46V
It is feasible, as Ecell 0 is positive, ∴ ?G0 is negative.
- (iii) The electrode reaction is written as,
- 2Fe3+ (aq) + 2Br- (aq)→ 2Fe2+ (aq) + Br2
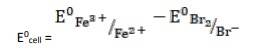 k
k= 0.77V - 1.09V
∴ E0cell = - 0.32 V
It is not feasible, as E0cell is negative, ∴ ?G0 is positive.
- (iv) The electrode reaction is written as,
- Ag(s) + Fe3+ (aq) → Fe2+ (aq) + Ag+ (aq)
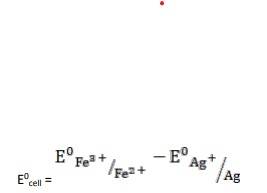
= 0.77V - 0.80V
∴ E0cell = -
New answer posted
6 months agoContributor-Level 10
14.16
Starch consists of two components – amylase and amylopectin. Amylose is a long linear chain of α–D-(+)-glucose units joined by C1-C4glycosidic linkage (α -link).
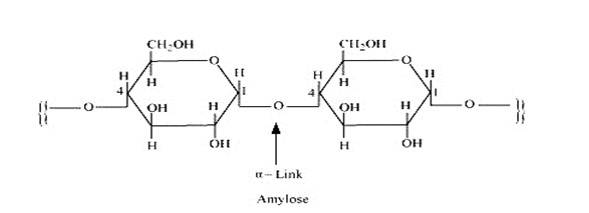
Amylopectin is a branched-chain polymer of –D-glucose units, in which the chain is formed by C1-C4 glycosidic linkage and the branching occurs by C1-C6 glycosidic linkage.

On the other hand, cellulose is the main structural material of tree and other plants. Wood is 50% cellulose, while cotton wool is almost pure cellulose. It is linear chain natural polymers of β-D-glucose units joined by 1, 4-glycosidic linkage (natural linear polymers).

New answer posted
6 months agoContributor-Level 10
14.15
Hydrolysis is the process of using water to break down a molecule into two parts. It is usually a type of decomposition reaction where one reactant is water, where water is used to break chemical bonds in the other reactant. It can be considered as reverse of a condensation reaction.
The general formula of a hydrolysis reaction is:
XY + H2O → XH + YOH
(i) On hydrolysis with dilute acids, sucrose yields an equimolecular mixture of α –D glucose and β–D- fructose.
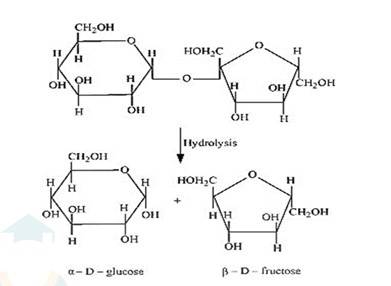
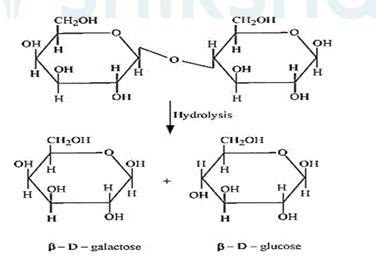
(ii) The hydrolysis of lactose gives β–D-galactose and β–D-glucose as final products.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers