Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
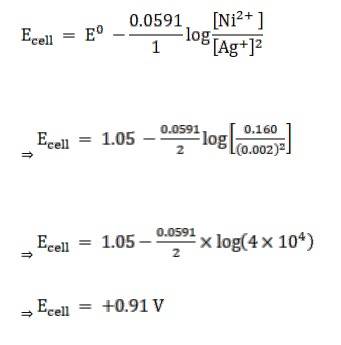
Given:
[Ag+] = 0.002 M
[Ni2+] = 0.160 M
n = 2
(n = moles of e- from balanced redox reaction)
E0cell= 1.05 V
Now, using the Nernst equation, we get,
New answer posted
6 months agoContributor-Level 10
Given:
For hydrogen electrode, pH = 10
n = 1
(n = moles of e- from balanced redox reaction)
On using the formula [H+] = 10– pH
⇒ [H+] = 10 − 10 M
We know,
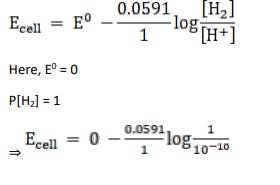
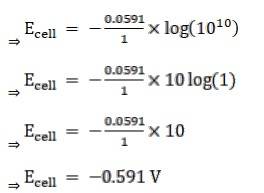
New answer posted
6 months agoContributor-Level 10
For a substance to oxidise Fe2+ to Fe3+ ion, it must have high reduction potential than Fe3+. The reduction potential of Fe3+ to Fe2+ reaction is 0.77V, the substances which have reduction potentials higher than this value will oxidise Fe2+ ions. Comparing the values, from the table:

New answer posted
6 months agoContributor-Level 10
NO, because Zn is very reactive with Cu. It reacts with copper sulphate to form zinc sulphate i.e., Zn displaces Cu and metallic Cu is also formed.
The reaction is given as:
Zn + CuSO4 ⇒ ZnSO4 + Cu
New answer posted
6 months agoContributor-Level 10
To determine the standard electrode potential of the system Mg2+|Mg, connect it to the standard hydrogen electrode (SHE). Keep the Mg2+|Mg system as cathode and SHE as cathode. This is represented as shown below.
Pt (s) | H2 (g, 1 bar)| H+ (aq, 1 M) |Mg2+ (aq, 1M)| Mg
The electrode potential of a cell is given by
E? = E? R – E? L
Where,
E? R- Potential of the half-cell in the right side of the above representation
E? L- Potential of the half-cell in the left side of the above representation
It is to be noted that the potential of the standard hydrogen electrode is zero.
Therefore, E? L = 0
E? = E? R – 0
⇒ E? = E? R
New answer posted
6 months agoContributor-Level 10
4.3 The order of the reaction is sum of the powers of concentration of reactants in the rate law. According to this,
The order of the reaction = 1/2 + 2
= 2 1/2 or 2.5
The order of the reaction is 2.5
New answer posted
6 months agoContributor-Level 10
4.2 Given-
Initial concentration (A1) = 0.5M
Final concentration (A2) = 0.4M
Time = 10 mins.
The formula for average rate of the reaction is,
rav = -1/2 X Δ{A} / Δt → Equation 1
? {A} = (A2)-( A1), the equation 1 is written as,
rav = -1/2 X A2 - A1 / Δt
= -1/2 X 0.4-0.5 / 10
= -1/2 X 0.1 / 10
= 0.005 mol L-1 min-1
= 5 * 10-3 mol L-1 min-1
The average rate of the reaction is 5 * 10-3 mol L-1 min-1
New answer posted
6 months agoContributor-Level 10
4.1 Given-
Initial concentration (R1) = 0.03M
Final concentration (R2) = 0.02M
Time = 25 mins.
The formula for average rate of the reaction is,
rav = - Δ{R} / Δt → Equation 1
? {R} = (R2)-( R1), the equation 1 is written as,
rav = - R2 - R1 / Δt
= -0.02-0.03 / 25
= 4 X 10-4 mol L-1 min-1
The average rate of reaction in seconds is given by,
= 4 X 10-4 mol L-1 / 60 S
(dividing by 60 to convert minutes to seconds)
= 6.6 * 10-6 mol L-1 s-1
The average rate of the reaction in minutes is 4 * 10-4 mol L-1 min-1 and in seconds is 6.6 * 10-6 mol L-1 s-1
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
14.1
Glucose and sucrose are carbohydrates (optically active polyhydroxy aldehydes or ketones).
Structure of glucose:
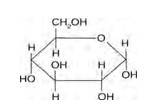
Structure of sucrose:
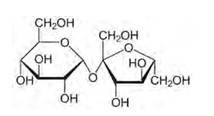
As you can see both the compounds have five –OH and eight –OH groups respectively. These –OH groups are responsible for the extensive hydrogen bonding with water. This –H bonding is responsible for the solubility of glucose and sucrose in water.
In case of cyclohexane or benzene (simple six-membered ring compounds), they do not contain any – OH groups. Hence, they cannot undergo –H bonding with water and are insoluble.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
