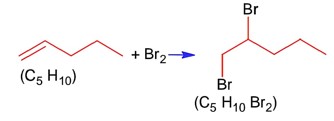
Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
Biomolecules are organic compounds essential for life; they are found in all living organisms. In chemistry class 12, we talked about the following biomolecules: carbohydrates, proteins, lipids, nucleic acids, enzymes, and vitamins.
Biomolecules chemistry class 12 connects biology and chemistry and helps us understand metabolism, heredity, and biochemical reactions.
New answer posted
3 months agoContributor-Level 9
You must use the NCERT for the CBSE Class 12 board exams. The NCERT textbook is enough for understanding the Biomolecules. All concepts, definitions, and reactions asked in the exams are directly covered in the textbook. We have provided NCERT Solutions for Class 12 Chemistry, Biomolecules, as well as for better practice.
However, for competitive exams like NEET and JEE, while NCERT remains an important resource, students should also solve additional questions from reference books like MTG NCERT at Your Fingertips, NCERT Exemplar, or practice modules by coaching institutes. These help build speed, accuracy, and deeper conceptual clarity
New answer posted
3 months agoContributor-Level 10
Nitrogen, sulphur and halogens are tested in an organic compound by lassaigne's test.
The organic compound is fused with sodium metal as to convert these elements into ionisable inorganic substances.
Na + C + N → NaCN
2Na + S → Na? S
2Na + X? → 2NaX
The cyanide, sulphide or halide ions can be confirmed in aqueous solution by usual test.
New answer posted
3 months agoContributor-Level 10
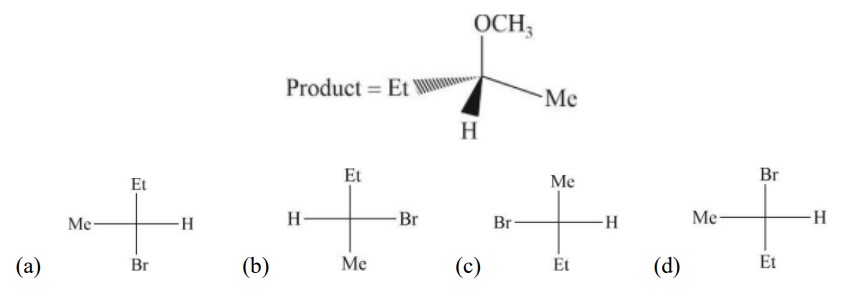
In S?2 reaction, inversion takes place, so configuration get reversed.
New answer posted
3 months agoContributor-Level 10
When Ferric chloride reacts with potassium thiocyanate a blood red colour of Ferric thiocyanate is formed
FeCl? + 3KSCN → Fe (SCN)? + 3KCl
New answer posted
3 months agoContributor-Level 10
To stop bleeding, FeCl? is applied locally because FeCl? causes denaturation of proteins present in blood. It is a case of coagulation.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers