Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Density,
Cell edge, a = 0.4518nm = 0.458 * 10-7 cm
Effective number of atoms, Z = 4 for CCP.
Using ;
A = 105.79 g/mol
Ans. is 106 (the nearest integers)
New answer posted
3 months agoContributor-Level 10
Reducing power for group-15 hydrides increases down the group, so BiH3 is the strongest reducing agent.
New answer posted
3 months agoContributor-Level 10
Sulphide ion (s2-) form ores commonly with Pb & Ag as PbS and Ag2S
New answer posted
3 months agoContributor-Level 10
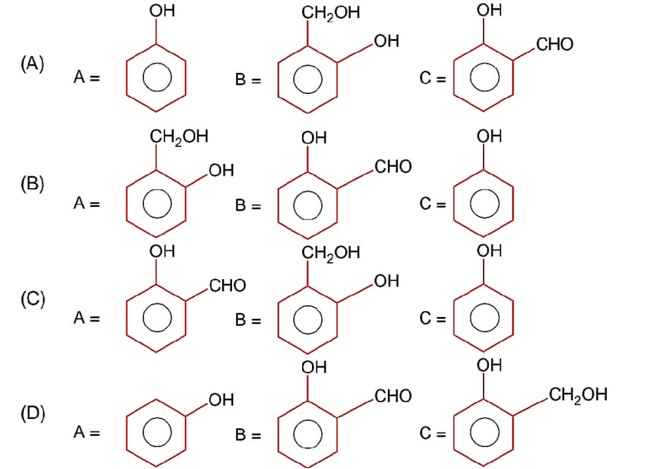
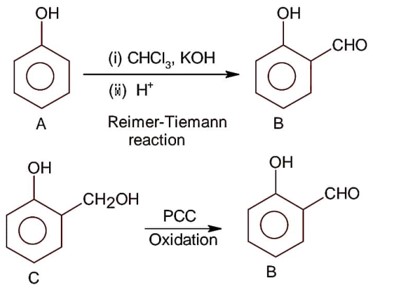
Compound A is phenol since phenol gives dark green colour with FeCl3.
New answer posted
3 months agoContributor-Level 10
Depression in freezing point will be maximum for Al2 (SO4)3 since its Van't Hoff factor is the highest. So its solution will have the lowest freezing point.
New answer posted
3 months agoContributor-Level 10
When AgNO3 is added to Kl solution, precipitate of Agl is formed which adsorb I- ion from
Dispersion medium to give negatively charged sol
Agl/l-- negatively charged sol.
New answer posted
3 months agoContributor-Level 10
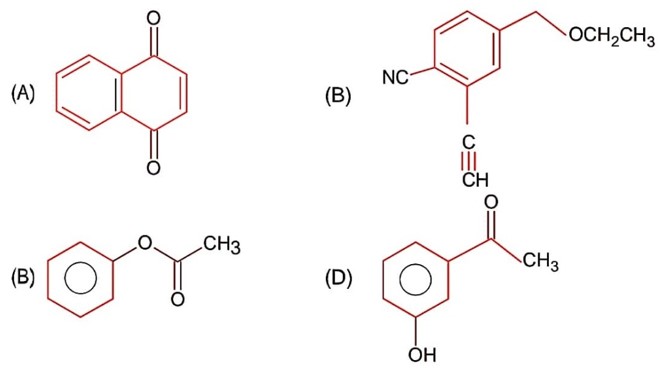
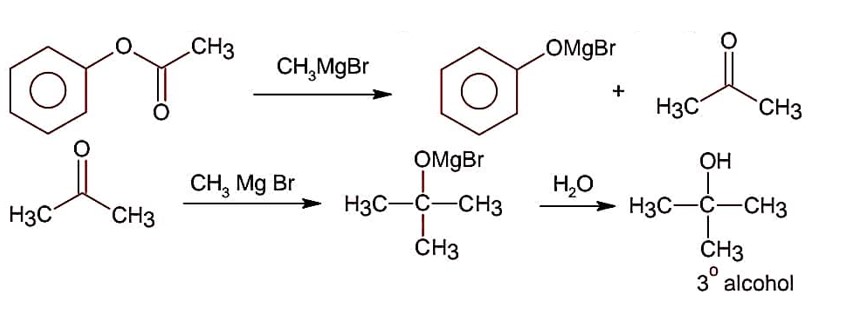
Esters on treating with excess CH3MgBr followed by hydrolysis gives 3° alcohol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers