Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
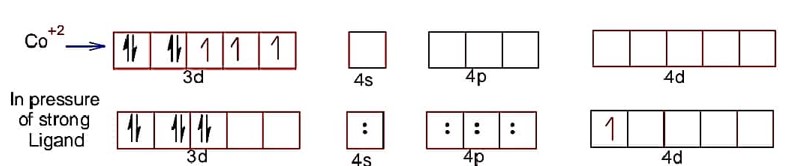
Hence, electronic configuration of CO2+ is
spin magnetic moment =
New answer posted
3 months agoContributor-Level 10
Both statement are correct for the glass body heating, but reason is not correct explanation during heating process of glass, constituents unit rupture of glass body and gives the edge smoothness.
New answer posted
3 months agoContributor-Level 10
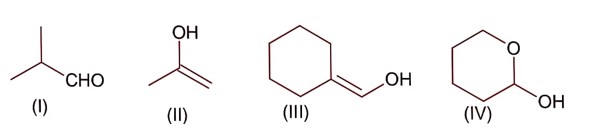
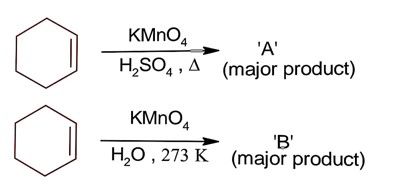
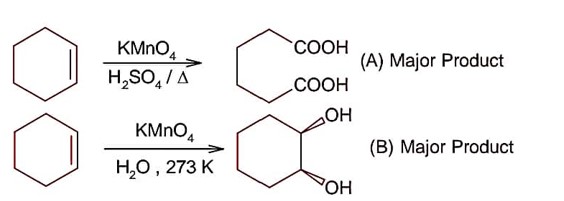
All structure can produce -CHO functional group which gives +ve test of Tollen's reagent except structure (II), because if forms ketonic group after tautomerisation
New answer posted
3 months agoContributor-Level 10
Deep blue colour solution
In cation analysis of Cu+ ions, precipitate formed is CuS on treating with H2S and HCl which dissolved in HNO3 and produced blue colour complex solution
New answer posted
3 months agoContributor-Level 10
In
New answer posted
3 months agoContributor-Level 10
In fcc structure of diamond four C present in fcc lattice and other four C present in tetrahedral voids where 50% of tetrahedral voids are occupied. Hence number of carbon atoms present per unit cell of diamond is 8.
New answer posted
3 months agoContributor-Level 10
Complex has Ni4+ and strong ligand, hence following are the metal ion electronic configuration
Change of unpaired electron = 2
New answer posted
3 months agoContributor-Level 10
Wt of Cl- in 100 ml = 1.8 * 10-3 gm
Mol. of Cl- in 100 ml =
i.e. 0.507 milli mole in one lit required in one hr.
Coagulation value = (millimole/lit) required in one hr = 0.507
= 1 (the nearest integer)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers