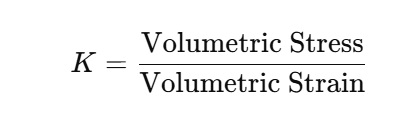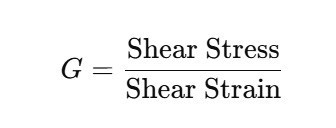NCERT
Get insights from 620 questions on NCERT, answered by students, alumni, and experts. You may also ask and answer any question you like about NCERT
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoBeginner-Level 5
Mechanical Properties of Fluids holds significant importance in both school-level examinations and competitive exams like NEET and JEE.
- In the CBSE Class 11 Physics annual examination, this chapter typically carries a weightage of 3 to 5 marks.
- In NEET, 1 to 2 questions are usually asked from this chapter, focusing on fluid pressure, Bernoulli's principle, and surface tension.
- JEE Main occasionally includes a conceptual or numerical question based on fluid dynamics or viscosity.
Students must focus on understanding Class 11 Physics Mechanical Properties of Fluid thoroughly helps students build a strong conceptual base for advanced physics
New answer posted
8 months agoBeginner-Level 5
The Class 11 Physics Mechanical Properties of Fluids is a foundational topic that introduces students to the behavior of fluids under various forces. It holds significant importance in both school-level examinations and competitive exams like NEET and JEE. In the CBSE Class 11 Physics annual examination, this chapter typically carries a weightage of 3 to 5 marks. Question generally asked are realted to fluid pressure, Bernoulli's principle, and surface tension. We have provided NCERT Solutions for Class 11 Physics Mechanical Properties of Fluids, Students can take help of the provided solutions for revising concepts, studying and
New answer posted
8 months agoContributor-Level 10
As the name suggests, a p-n junction diode means an n-type and p-type semiconductor joined together. At the junction, they form a depletion region. In forward bias where n is connected to negative and p to positive, the barrier potential reduces, allowing the current to flow. In reverse bias, there is a tiny leakage but the current is blocked and the barrier increases. This one-way behaviour is ideal for signal demodulation, rectification (converting AC to DC) and voltage regulation. They are the basic or fundamental components in logic gates, power supplies and communication systems offering efficiency, high-speed and reliability.
New answer posted
8 months agoBeginner-Level 5
Students ask this question many a times that wehter NCERT are enough or they should follow other reference books. Students should use NCERT books for CBSE Class 12 board exams, NCERT is more than sufficient for mastering the Biomolecules chapter. All key concepts, definitions, and reactions asked in the exams are directly covered in the textbook, WE have provided NCERT Solutions for Class 12 chemistry Biomolecules.
However, for competitive exams like NEET and JEE, while NCERT remains the primary reference, students should also solve additional MCQs from reference books like MTG NCERT at Your Fingertips, NCERT Exemplar, or pra
New answer posted
8 months agoBeginner-Level 5
Chapter 10 Biomolecules of Class 12 Chemistry includes several important topics which are frequently asked in the state and cbses board exams. The most important topics in the Biomolecules chapter include the classification and structure of carbohydrates (monosaccharides, disaccharides, polysaccharides), amino acids and proteins (including peptide bond formation), enzymes and their characteristics, nucleic acids (DNA and RNA structure and components), and vitamins with their types and deficiencies.
Questions often focus on structural identification, naming, and functions of biomolecules, as well as simple reactions like hydrolysis
New answer posted
8 months agoBeginner-Level 5
Biomolecules are organic compounds essential to life, found in all living organisms. Biomolecules include carbohydrates, proteins, lipids, nucleic acids, enzymes, and vitamins.
NCERT Class 12 Chemistry chapter 10 Biomolecules covers the molecular basis of life, including the structure, types, and functions of each biomolecule. This chapter is important because it connects chemistry to biology and helps students understand metabolism, heredity, and biochemical reactions. It also forms the foundation for higher studies in medicine, biochemistry, and biotechnology. Biomolecules are also frequently tested in CBSE board exams and compet
New answer posted
8 months agoBeginner-Level 5
Amines are prepared by replacing the hydrogen atom with alkyl or aryl groups from amonia (NH? ). Students can differntiate Aliphatic and aromatic amines differ based on the type of group attached to the nitrogen atom.
- Aliphatic amines have one or more alkyl groups (like methyl or ethyl) bonded to the nitrogen, such as ethylamine (CH? NH? ).
- Aromatic amines have an aryl group (like benzene) directly bonded to nitrogen, for example, aniline (C? H? NH? ).
Aliphatic amines are generally more basic than aromatic amines because, in aromatic amines, the lone pair on nitrogen is delocalized into the aromatic ring through resonanc
New answer posted
8 months agoBeginner-Level 5
Chapter 9 Amines of class 12 chemistry is really important part of organic chemistry. Several questions are asked related to Amine preparation methods in CBSE Boards and other state board exams. Here are several methods to prepare Amine in labs, check below;
Reduction of nitro compounds, nitriles, and amides
Ammonolysis of alkyl halides
Gabriel Phthalimide synthesis
Hoffmann bromamide degradation
Students can check the ncert solutions for Class 12 Chemistry Amines, to better understand the question types and concepts asked in the exams.
New answer posted
8 months agoContributor-Level 10
According to the NCERT Solutions for Class 11 Physics Chapter 8 Mechanical Properties of Solids, when an external force is applied to a solid, the solid undergoes changes in shape or size which is called deformation. The solid deformation can be classified into:
Elastic Deformation: It is when the body returns to its original size and shape after the force is removed. It occurs within the elastic limit and follows Hooke's law. The most common examples include stretching a helical spring or a rubber band.
Plastic Deformation: It occurs beyond the elastic limit and here after the removal of the force, the body does not return to its origin
New answer posted
8 months agoContributor-Level 10
The modulus of elasticity refers to the material's capacity to withstand or resist deformation when stress is applied. There are three types of the modulus of elasticity including:
Young's Modulus (E): It is used to measure a solid's elasticity when longitudinal stress (tensile or compressive) is applied to it. Mathematically, it is represented by the following formula;
SI Unit is N/m² (Pascal, Pa).
Bulk Modulus (K): It is used to measure the ability of the material to resist volume change when uniform pressure is applied to it. Mathematically, it is:
Shear Modulus (G) or Modulus of Rigidity: It is used when a shear
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers