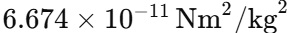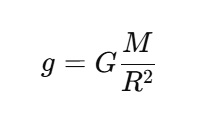NCERT
Get insights from 620 questions on NCERT, answered by students, alumni, and experts. You may also ask and answer any question you like about NCERT
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoBeginner-Level 5
Students studying class 12 chemistry must have clear understanding of the basic knowledge. Students must be aware of physical properties of these compunds. Aldehydes, ketones, and carboxylic acids generally have higher boiling points than hydrocarbons of similar molecular weight due to dipole-dipole interactions and hydrogen bonding (especially in carboxylic acids). Lower members are soluble in water, but solubility decreases with increasing molecular weight.
New answer posted
8 months agoContributor-Level 10
In Newton's Law of Gravitation, G (Universal Gravitational Constant) has a fixed value as it is a fundamental constant which is:
G determines the strength of gravitational interactions and it remains the same everywhere in the universe. It plays a significant role in cosmology and astrophysics and it is important in calculating the forces between celestial bodies. Due to the gravitational pull of a massive body like Earth, 'g' (Acceleration due to Gravity) is the acceleration which the object experiences. The value of g changes based on the planetary composition, latitude, and altitude. G is universal and g is dependent on the location.
New answer posted
8 months agoBeginner-Level 5
Students can access the Class 12 Chemistry Aldehyde Ketone and Carboxylic Acids NCERT Solutions for all chapters in our pages. We also provide PDF of NCERT Solutions of all chapter for free. Students can use following steps to download Aldehyde Ketone and Carboxylic Acids NCERT Solution PDF;
- Visit " NCERT Solutions for Class 12 Chemistry Aldehyde Ketone and Carboxylic Acids" page
- Go to heading " Class 12 Chemistry Aldehyde Ketone and Carboxylic Acids NCERT Solutions PDF"
- Click on the link-" Download Free PDF"
Your PDF is ready to use, Students can use the Aldehyde Ketone and Carboxylic Acids NCERT Solutions PDF for various purposes includi
New answer posted
8 months agoBeginner-Level 5
Class 12 Chemistry Chapter 8 Aldehydes, Ketones, and Carboxylic Acids consists important topics such as the structure, nomenclature, preparation, and chemical properties of these important organic compounds. Class 12 Chemistry Chapter 8 covers key reactions like nucleophilic addition, oxidation, reduction, aldol condensation, and Cannizzaro reaction. The acidic nature and reactivity of carboxylic acids are also discussed in NCERT Chemistry Chapter 8 Aldehydes, Ketones, and Carboxylic Acids. These compounds have vast applications in medicines, perfumes, food preservatives, and industrial solvents.
To help understand the topic, we have pr
New answer posted
8 months agoContributor-Level 10
It is due to a concept taken from Newton's Law of Gravitation. It says that each mass element of the shell puts an attractive force on a particle inside but due to the fact that the shape of the shell is symmetrical, these forces cancel out in every direction.
To prove it mathematically, one can use Gauss's Law for Gravitation. It states that inside a uniformly distributed spherical shell, the net gravitational field is zero. It differs from electrostatics, which states that a conducting shell blocks or stops the external electric fields. The shielding concept is not part of gravitation where the external bodies exert a gravitational fo
New answer posted
8 months agoContributor-Level 10
Gravitational Potential Energy (U) is the energy an object has due to its position in a gravitational field. It is defined as:
Where M represents the mass of the larger body, m is the mass of the smaller body, r is the distance between them, and G stands for the gravitational constant.
G is written with a negative sign to show that the gravitational potential energy is always lower than zero and the values even decrease as the objects move apart from each other. It implies that to separate two masses, work should be done against the gravity. At infinity, U = 0, implying that no external force is required to keep them apart. When two obj
New answer posted
8 months agoContributor-Level 10
According to Newton's Law of Universal Gravitation, each object in the universe attracts every other object with a force that depends on the distance between them and their masses. This force is mathematically given by the following formula:
Here, F is the gravitational force, G is the gravitational constant and m? and m? refers to the masses of the objects, and r represents the distance between them.
Newton's Law of Universal Gravitation explains the behaviour of tides due to the Moon's gravity, planetary motion, and even how satellites orbit the Earth. This law is widely used in space research and astrophysics to find out the mas
New answer posted
8 months agoBeginner-Level 5
Class 12 Chemistry Aldehydes Ketones and Carboxylic Acids is one of the most important organic chemistry topics in CBSE Class 12 Chemistry and competitive exams like JEE and NEET. The Chapter deals with the structure, properties, preparation, and reactions of aldehydes, ketones, and carboxylic acids.
Organic Chemistry carries around 28-30% weightage in the board exam. Within this NCERT Class 12 Chemistry CH 8 Aldehydes, Ketones, and Carboxylic Acids contributes around 5-8 marks in the CBSE Boards and Other state board exams. 2-3 direct questions (approximately 8-12 marks) appears in JEE Mains from Chapter 8 Class 12 Chemistry.
New answer posted
8 months agoBeginner-Level 5
NCERT Class 12 Chemistry Chapter 7 Alcohols, Phenols, and Ethers, is highly significant for JEE Mains as it forms a fundamental part of organic chemistry. Chapter 7 Alcohol, Phenol, and Ethers is frequently asked in JEE Mains with 1-2 direct questions every year. Questions are asked aften in the form of Conversions & Reaction Mechanisms, Acidity and Basicity Comparisons, and Named Reactions. NCERT Solutions for Class 12 Chemistry Ch 7 can be very helpful resource, Studenst can use that for practicing, revision, and conceptual understanding. Students can check solutions link below;
New answer posted
8 months agoContributor-Level 10
Along with NCERT Solutions for Class 12 Physics Chapter 12 Atoms, students can refer to reference books like:
H.C. Verma's Concepts of Physics
D.C. Pandey's Understanding Physics
I.E. Irodov's Problems in General Physics.
Students can also use online educational platforms for video explanations, solved examples, and interactive problem-solving sessions. To improve the overall preparation students should also practise mock tests and sample papers to strengthen their conceptual understanding and improve their confidence before exams.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers