Polymers
Get insights from 155 questions on Polymers, answered by students, alumni, and experts. You may also ask and answer any question you like about Polymers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (D)
Explanation: Vulcanisation is a process used to improve the physical properties of natural rubber. This method involves heating a mixture of raw rubber, sulphur, and an appropriate additive to temperatures ranging from 373 K to 415 K. Sulphur forms cross links at the reactive sites of double bonds during vulcanisation, stiffening the rubber.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A), (B) and (D)
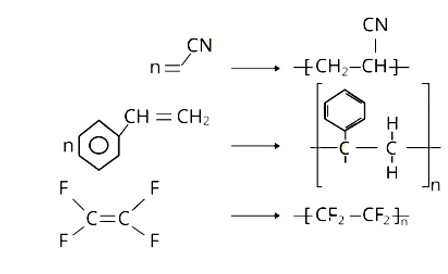
Explanation: Polyacrylonitrile is formed by the addition polymerisation of acrylonitrile in the presence of a peroxide catalyst. Polyacrylonitrile is used as a wool substitute in the manufacture of commercial fibers such as orlon or acrilan.
Polystyrene has the monomer unit styrene.
Teflon is made by heating tetrafluoroethene at high pressures with a free radical or persulphate catalyst.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (D)
Explanation: Polyamides: These polymers with amide linkages are important examples of synthetic fibers and are known as nylons.
Polyesters: These are the polycondensation products of dicarboxylic acids and diols.
Dacron or terylene is the most well-known example of a polyester.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (C)
Explanation: Synthetic rubbers are either homopolymers or copolymers of 1,3-butadiene or its derivatives with another unsaturated monomer. Polychloroprene or neoprene and Buna-N are examples of synthetic rubber.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (B)
Explanation: The polymers that are not harmful to the environment are biodegradable polymers. PHBV (Poly beta-hydroxybutyrate co-beta-hydroxy valerate) is a biodegradable polymer. It undergoes bacterial degradation in the environment. PHBV is obtained by the polymerization of 3-hydroxybutyric acid and 3-hydroxypentanoic acid.
Nylon-2, Nylon-6 polymers are also biodegradable. It is obtained by the polymerization of glycine and aminocaproic acid.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (D)
Explanation: Condensation polymers are formed by the addition of more than one monomeric unit. Bakelite and Melamine formaldehyde resin are condensation polymers. Bakelite is a polymer formed from phenol and formaldehyde. Melamine formaldehyde resin is a polymer formed from melamine and formaldehyde.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (D)
Explanation: The formation of addition polymers is accomplished through the repeated addition of monomer molecules with double or triple bonds, such as polythene from ethene and polypropene from propene. Homopolymers are addition polymers formed by the polymerisation of a single monomeric species.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (C) and (D)
Explanation: Fibers are thread-forming solids. They possess high tensile strength and high modulus. They have strong intermolecular forces of attraction like hydrogen bonding. Nylon and Terylene are used as fibers.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (D)
Explanation: Thermoplastic polymers are linear or slight branched-chain polymers. This type of polymer softens on heating and hardens on cooling. For example, Teflon and polystyrene are thermoplastic polymers.
So, options (A) and (D) are correct.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Option (A) and (C)
Explanation: Thermosetting polymers are cross linked polymers. These polymers on heating undergo cross linking in moulds. They become infusible on heating so cannot be reused again.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers