Redox Reactions
Get insights from 164 questions on Redox Reactions, answered by students, alumni, and experts. You may also ask and answer any question you like about Redox Reactions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
(a) Toluene can be oxidised to benzoic acid in acidic, basic and neutral media according to the following redox equations:
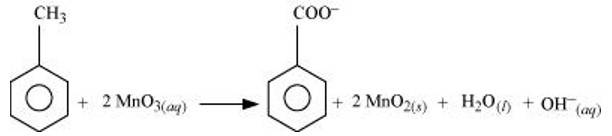
In the laboratory, benzoic acid is usually prepared by alcoholic KMnO4 oxidation of toluene. However, in industry alcoholic KMnO4 is preferred over acidic or alkaline KMnO4 because of the following reasons:
(i) The cost of adding an acid or the base is avoided because in the neutral medium, the base (OH- ions) are produced in the reaction itself.
(ii) Since reactions occur faster in homogeneous medium than in heterogeneous medium, therefore, alcohol helps in mixing the two reactants, i.e., KMnO4
New answer posted
8 months agoContributor-Level 10
The three examples are:
(i) When excess P4? (reducing agent) reacts with F2? (oxidizing agent), PF3? is produced in which P has +3 oxidation number.
P4? (excess) + F2? → PF3?
But if fluorine is in excess, PF5? is formed in which P has oxidation number of +5.
P4? ? + F2? (excess) → PF5?
(ii) Oxidizing agent is oxy
New answer posted
8 months agoContributor-Level 10
In AgF2, oxidation state of Ag is +2 which is very unstable. Therefore, it quickly accepts an electron to form the more stable +1 oxidation state.
Ag2+ + e– →Ag+
Therefore, AgF2, if formed, will act as a strong oxidising agent.
New answer posted
8 months agoContributor-Level 10
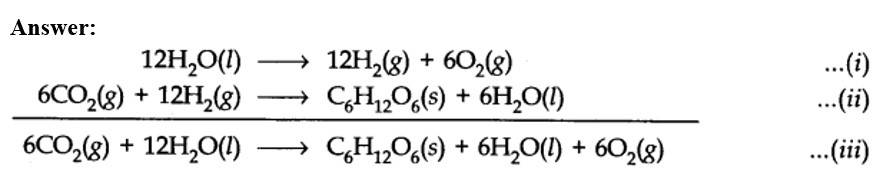
(a) Therefore, it is more appropriate to write the equation for photosynthesis as (iii) because it emphasises that 12H2O are used per molecule of carbohydrate formed and 6H2O are produced during the process.
(b) The purpose of writing O2 two times suggests that O2 is being obtained from each of the two reactants.
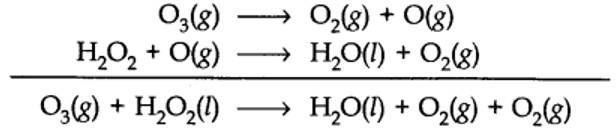
(a) or by using H2O218 or O318in reaction (b).
New answer posted
8 months agoContributor-Level 10
(i) In SO2, O.N. of S is +4. In principle, S can have a minimum O.N. of -2 and maximum of +6. Therefore, S in SO2 can either decrease or increase its O.N. and hence can act both as an oxidising as well as a reducing agent.
(ii) In H2O2, the O.N. of O is -1. In principle, O can have a minimum O.N. of -2 and maximum of zero (+1 is possible in O2F2 and +2 in OF2). Therefore, O in H2O2 can either decrease its O.N. from -1 to -2 or can increase its O.N. from -1 to zero. Therefore, H2O2 acts both as an oxidising as well as a reducing agent.
(iii) In O3, the O.N. of O is zero. It can only decrease its O.N. from zero to -1 or -
New answer posted
8 months agoContributor-Level 10
Substance | Oxidation number of C | Substance | Oxidation number of N |
CH2? Cl2? | 0 | N2? | 0 |
FC≡CF | +1 | N2? O | +1 |
HC≡CH | -1 | N2? H2? | -1 |
CHCl3? , CO | +2 | NO | +2 |
CH3? Cl | -2 | N2? H4? | -2 |
Cl3? C−CCl3? | +3 | N2? O3? | +3 |
H3? C−CH3? | -3 | NH3? | -3 |
CCl4? , CO2? | +4 | NO2? | +4 |
CH4? | -4 | N2? O5? | +5 |
New answer posted
8 months agoNew answer posted
8 months agoContributor-Level 10
(a) H2? SO5? by conventional method.
Let x be the oxidation number of S
2 (+1) + x + 5 (−2) = 0
x = +8
+8 oxidation state of S is not possible as S cannot have an oxidation number more than 6. The fallacy is overcome if we calculate the oxidation number from its structure HO−S (O2)−O−O−H.
−1+X+2 (−2)+2 (−1)+1=0
x=+6
(b) Dichromate ion
Let x be the oxidation number of Cr in dichromate ion
2x+7 (−2)=−2
x=+6
Hence the oxidation number of Cr in dichromate ion is +6. This is correct and there is no fallacy.
(c) Nitrate ion, by conventional method
Let x be the oxidation number of N in nitrate ion.
x+3 (−2)=−1
From the
New answer posted
8 months agoContributor-Level 10
Writing the O.N. of each atom above its symbol, we have,
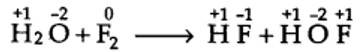
here, the O.N. of F decreases from 0 in F2 to -1 in HF and increases from 0 in F2 to +1 in HOF. Therefore, F2 is both reduced as well as oxidised. Thus, it is a redox reaction and more specifically, it is a disproportionation reaction.
New answer posted
8 months agoContributor-Level 10
(a) Here, O is removed from CuO, therefore, it is reduced to Cu, while O is added to H2 to form H2O, therefore, it is oxidised. Further, O.N. of Cu decreases from +2 in CuO to 0 in Cu but that of H increases from 0 in H2 to +1 in H20. Therefore, CuO is reduced to Cu but H2 is oxidised to H2O. Thus, this is a redox reaction.
(b) Here O.N. of Fe decreases from +3 in Fe2O3 to 0 in Fe while that of C increases from +2 in CO to +4 in CO2. Further, oxygen is removed from Fe2O3 and added to CO, therefore, Fe2O3 is reduced while CO is oxidised. Thus, this is a redox reaction.
(c) Here, O.N. of B decreas
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
