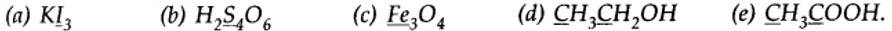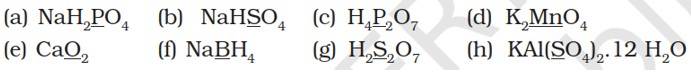Redox Reactions
Get insights from 164 questions on Redox Reactions, answered by students, alumni, and experts. You may also ask and answer any question you like about Redox Reactions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
(a) In Kl3, since the oxidation number of K is +1, therefore, the average oxidation number of iodine = -1/3. But the oxidation number cannot be fractional. Therefore, we must consider its structure, K+ [I —I < I]–. Here, a coordinate bond is formed between I2 molecule and I– ion. The oxidation number of two iodine atoms forming the I2 molecule is zero, while that of iodine forming the coordinate bond is -1. Thus, the oxidation number of the three I atoms, atoms in Kl3 is 0, 0 and -1, respectively.
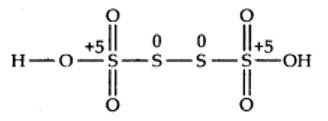
(b) By conventional method O.N. of S in H2S4O6is calculated as:
2 (+1) +4x + 6) (-2) = 0
Or x = +2.5
But all the four
New answer posted
8 months agoContributor-Level 10
Let x be the oxidation number to the underlined elements in the given species:
(a) NaH2PO4
(+1) + 2 (+1) + x + 4 (-2) = 0
x + 3 – 8 = 0
x = +5
(b) NaHSO4
(+1) + (+1) + x + 4 (-2) = 0
x – 6 = 0
x = +6
(c) H4P2O7
4 (+1) + 2x + 7 (-2) = 0
2x -10 =0
x = +5
(d) K2MnO4
2 (+1) + x + 4 (-2) = 0
x – 6 = 0
x = +6
(e) CaO2
2 + 2x = 0
x = -1
(f) NaBH4
1 + x + 4 (-1) = 0 (Since H is present as hydride ion.)
x = +3
(g) H2S2O7
2 (+1) + 2x + 7 (-2) = 0
x = +6
(h) KAl (SO4)2.12H2O
+1 + 3 + 2x + 8 (-2) + 12 (2 x 1 - 2) = 0
x = +6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers