Solid State
Get insights from 127 questions on Solid State, answered by students, alumni, and experts. You may also ask and answer any question you like about Solid State
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
1.15 Number of atoms in close packaging = 0.5 mol
1 atom has 6.022*1023 particles
So Number of particles in close-packed = 0.5 * 6.022 * 1023 = 3.011*1023
Number of tetrahedral voids = 2 * number of atoms in close packaging
Number of tetrahedral voids = 2 * 3.011 * 1023= 6.022*1023
Number of octahedral voids = number of atoms in close packaging
So the number of octahedral voids = 3.011 * 1023
Total number of voids = Tetrahedral void + octahedral void
=6.022 * 1023 + 3.011 * 1023= 9.03*1023
New answer posted
9 months agoContributor-Level 10
1.14 In square close-packed layer, a molecule is in contact with four of its neighbours. Therefore, the two-dimensional coordination number of a molecule in square close-packed layer is 4.
New answer posted
9 months agoContributor-Level 10
1.13 (i) An atom located at the corner of a cubic unit cell is shared by eight adjacent unit cells. Therefore, 1/8th portion of the atom is shared by one unit cell.
(ii) An atom located at the body centre of a cubic unit cell is not shared by its neighbouring unit cell. Therefore, the atom belongs only to the unit cell in which it is present i.e., its contribution to the unit cell is 1
New answer posted
9 months agoContributor-Level 10
1.12

(ii) Face-centered unit cell: In a face-centered unit cell, the constituent particles are present at the corners
and one at the centre of each face.
End-centered unit cell: An end-centered unit cell contains particles at the corners and one at the centre of
any two opposite faces.
New answer posted
9 months agoContributor-Level 10
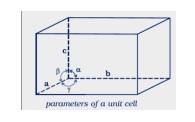
1.11 The six parameters that characterize a unit cell are as follows.
(i) Its dimensions along the three edges, a, b, and c these edges may or may not be equal.
(ii) Angles between the edges these are the angles (between edges b and c), (between edges a and c), and (between edges a and b)
New answer posted
9 months agoContributor-Level 10
1.10 The significance of a lattice point is that each lattice point represents one constituent particle of a solid which may be an atom, a molecule (group of atom), or an ion.
New answer posted
9 months agoContributor-Level 10
1.9 Metallic solids are electrical conductors, malleable, and ductile.
New answer posted
9 months agoContributor-Level 10
1.8 In solid state, ions are held together by strong electrostatic forces and are not free to move about within the solid. In ionic compounds, electricity is conducted by ions. Hence, in molten state or in solution form, the ions are free to move and can conduct electricity
New answer posted
9 months agoContributor-Level 10
1.7 The given properties are the resource of a covalent or network solid. Therefore, the given solid is a covalent or network solid. Examples of such solid are quartz (SiO) and diamond (C).
New answer posted
9 months agoContributor-Level 10
1.6 Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite, rubidium, argon, silicon carbide. Solids have been classified into different categories based on chemical bonding. The chemical bonding depends on the intermolecular forces of attraction between the atoms.
a) Potassium sulphate →Ionic solid
b) Tin→ Metallic solid
c) Benzene→ Molecular (non-polar) solid
d) Urea→ Polar molecular solid
e) Ammonia→ Solid ammonia is a hydrogen-bonded molecular solid which is also known as polar molecular solid
f) Water→ Hydrogen bonded molecular solid
g) Zinc sulphide→ Ionic solid
h) Graphite→ Covalent or network so
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
