Thermodynamics
Get insights from 327 questions on Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Slope of the curve = f(V) , where V is the volume
Slope of P = f(V) curve at ((Po, V0 )= f(Vo)
Slope of adiabatic at (Po, V0 )= k(-Y)Vo-1-Y =-YPo/Vo
Now heat absorbed in the process P= f(V)
dQ=dU+dW= nCvdT+pdV
pV=nRT
T= pV/nR
T=
nCv
After solving we get
=
Heat is absorbed where dQ/dV>0 when gas expands
Hence YPo+Vof'(Vo)>0 or f'(Vo)>(-Y )
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
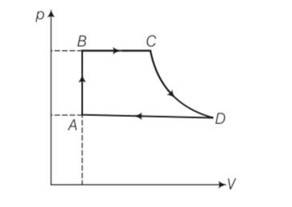
(a) For process AB
Volume is constant , hence work done dW=0
dQ=dU+dW=dU+0=dU
= nCvdT= nCv(TB-TA)
=
=
Heat exchanged =
(b) For process BC , p =constant
dQ= dU+dW =
heat exchanged =
(c) For process CD , because CD is adiabatic , dQ= heat exchanged =0
(d) DA involves compression of gas from VD to VA at constant pressure PA
heat transferred as similar way as BC1
hence dQ = PA(VA-VD)
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
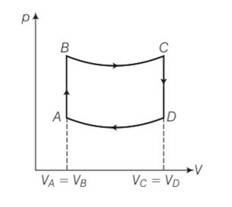
(a) For the process AB
dV=0 and dW=0
dQ=dU+dW=dU
dQ=dU= change in internal energy , so heat utilised is equal to change in internal energy.
Since p= in adiabatic temperature is directly proportional to pressure. So heat is supplied to the system in process AB.
(b) For the process CD volume is constant but the pressure decreases, hence temperature also decreases . so heat is also given to the surroundings.
(c) WAB= , WCD=
WBC=
= [pV]=
WDA=
B and C lies on adiabatic curve BC
PBVBY= PCVCY
PC = PB( )Y = PB( )Y= 2-YPB
Total work done by the engine in one cycle ABCD
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
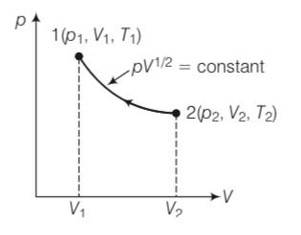
pV1/2= constant
P=k/
Work done from 1 to 2
W=
from ideal equation = pV=nRT
T= pV/nR=
T=
T1= , T1=
=
U=
= RT1( )
=2p1V11/2( )
= 2p1V11/2(2 )
= 2p1V1( )= 2RT1( )
=
= RT1( )+ 2RT1( )
=
New answer posted
8 months agoContributor-Level 10
Temperature inside the refrigerator, = 9 = 9 + 273 K = 282 K
Room temperature, = 36 = 36 + 273 = 309 K
Coefficient of performance = = = 10.44
Therefore, the coefficient of performance is 10.44
New answer posted
8 months agoContributor-Level 10
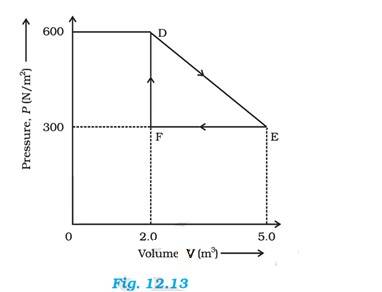
Total work done by the gas from D to E to F = Area of = EF
Where DF = Change in pressure = 600 – 300 = 300 N/
FE = change in volume = 5-2 = 3
Area of 3 = 450 J
Therefore work done by the gas from D to E to F is 450 J.
New answer posted
8 months agoContributor-Level 10
Heat is supplied to the system at a rate of 100W
Hence, heat supplied, Q = 100 J/s
The system performs at the rate of 75 J/s
Hence, work done, W = 75 J/s
From the 1st law of Thermodynamics, we have Q = U + W, where U is the internal energy
U = Q – W = 100 – 75 = 25 J/s = 25 W
Therefore the internal energy of the given electric heater increases at a rate of 25 W.
New answer posted
8 months agoContributor-Level 10
Work done by the steam engine per minute, W = 5.4 J
Heat supplied by the boiler, H = 3.6 J
Efficiency of the engine, = = = 0.15
Amount of heat wasted = Input energy – Output energy
= 3.6 5.4 J
New answer posted
8 months agoContributor-Level 10
(a) When the stopcock is opened, the volume became double between cylinders A and B. Since volume is inversely proportional to pressure, the pressure will become half. So the initial pressure of 1 atm in cylinder A will become ½ atm in cylinder A and B.
(b) The internal energy will change when there is work done by the gas. In absence of any work done, there will be no change in internal energy.
(c) In absence of any work done, there will be no change in the temperature.
(d) The given process is a case of free expansion. It is rapid and cannot be controlled. The intermediate states do not satisfy the gas equation an
New answer posted
8 months agoContributor-Level 10
The work done, W = 22.3 J
Being an adiabatic process, Q = 0
W = -22.3 J : since the work is done on the system
From the 1st law of thermodynamics, we know Q = W, where is the change of internal energy of the gas
U = 22.3 J
When the gas goes from state A to state B via a process, the net heat absorbed by the system is:
Q = 9.35 cal = 9.35 J = 39.1765 J
Heat absorbed Q = W
W = Q - = 39.1765 – 22.3 = 16.8765 J
Therefore, work done by the system is 16.8765 J
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers