Alcohols, Phenols and Ethers
Get insights from 16 questions on Alcohols, Phenols and Ethers, answered by students, alumni, and experts. You may also ask and answer any question you like about Alcohols, Phenols and Ethers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
At room temperature Hg is liquid and it is purified by 'Distillation method'.
New answer posted
5 months agoContributor-Level 10
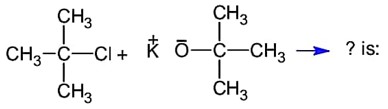
Base is tert-butoxide thus favours elimination with 3° alkyl halide and gives alkene
New answer posted
6 months agoContributor-Level 10
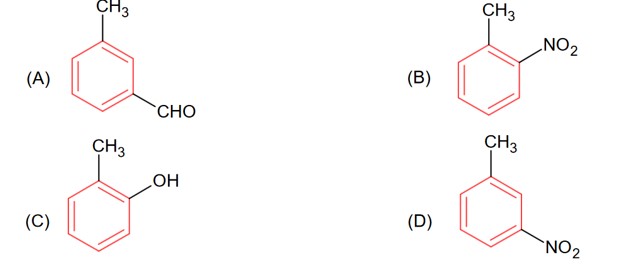
OH group is the more activating group and ortho-para director, hence ortho position attach will provide meta substituted product with respect to – CH3 in option (C).
New answer posted
6 months agoContributor-Level 10
Due to H- bond in water, it has high melting point and melting point of other hydrides of the group are depending upon the molecular weight.
New answer posted
6 months agoContributor-Level 10
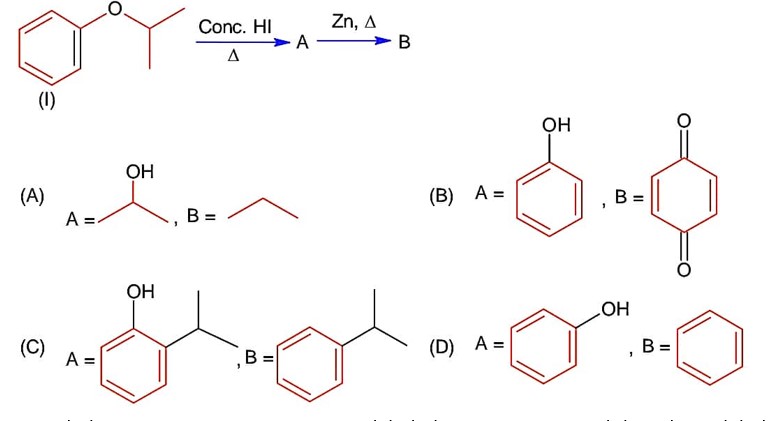
Phenol is weakly acidic compound but it is more acidic than alcohol and water because its conjugate base is more stable. Hence statement -I is correct while statement -II is wrong.
Related Tags
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers