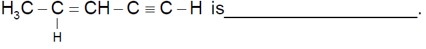Chemistry NCERT Exemplar Solutions Class 11th Chapter One
Get insights from 66 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter One, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter One
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 9
CrCl? ·3NH? ·3H? O gives 3 moles of AgCl precipitate. This means all three Cl? are outside the coordination sphere.
The complex is [Cr (NH? )? (H? O)? ]Cl?
The 3 chloride ions satisfy only primary valency.
secondary valency satisfied by chloride ion = 0
New answer posted
5 months agoContributor-Level 9
PV = nRT
n = PV/RT = (1 atm * 4*10? L) / (0.083 LatmK? ¹mol? ¹ * 300 K) = 1.6 * 10? mol
Mass = n * Molar Mass = 1.6 * 10? mol * 16 g/mol = 25.6 * 10? g ≈ 26 * 10? g
New answer posted
5 months agoContributor-Level 9
Energy per second = 1000 J / 10 s = 100 J/s
Energy of one photon E = hc/λ = (6.626*10? ³? * 3*10? ) / (400*10? ) = 4.965 * 10? ¹? J
Number of electrons ejected = Total energy / Energy per photon = 100 / (4.965 * 10? ¹? ) = 20.14 * 10¹? ≈ 2 * 10²?
New answer posted
5 months agoContributor-Level 9
A + B? 2C
Initial: 1, 1
At eq: 1-x, 1+2x
K = [C]²/ ( [A] [B]) = (1+2x)²/ (1-x)² = 100
(1+2x)/ (1-x) = 10
1+2x = 10-10x => 12x = 9 => x = 3/4
[C] = 1+2x = 1+2 (3/4) = 1+1.5 = 2.5M = 25 * 10? ¹M
New answer posted
5 months agoContributor-Level 9
Fe? ³ + e? → Fe? ² E° = 0.77V
Zn (s) → Zn? ² + 2e? ; E° = 0.76V
Cell reaction: 2Fe? ³ + Zn → 2Fe? ² + Zn? ² E°cell = 1.53V
Ecell = E°cell - (0.059/2)log ( [Zn? ²] [Fe? ²]²/ [Fe? ³]²)
1.5 = 1.53 - (0.06/2)log (1 * [Fe? ²]²/ [Fe? ³]²)
-0.03 = -0.03 log ( [Fe? ²]/ [Fe? ³])²
1 = log ( [Fe? ²]/ [Fe? ³])² => [Fe? ²]/ [Fe? ³] = 10
Let total iron = T. [Fe? ³] + [Fe? ²] = T. [Fe? ³] + 10 [Fe? ³] = T. 11 [Fe? ³] = T.
fraction of Fe? ³ = [Fe? ³]/T = 1/11 ≈ 0.09
This solution seems to differ from the image. Let's follow the image's steps.
log ( [Fe? ²]/ [Fe? ³])² = 1 => ( [Fe? ²]/ [Fe? ³])² = 10
[Fe? ²]/ [Fe?
New answer posted
5 months agoContributor-Level 9
Cr? O? ²? + 6Fe²? + 14H? → 2Cr³? + 6Fe³? + 7H? O
n-factor for Cr? O? ²? = 6, for Fe²? = 1
M.E Cr? O? ²? = M.E Fe²?
M? V? n? = M? V? n?
0.02 * 15 * 6 = M? * 10 * 1
M? = (0.02 * 15 * 6)/10 = 0.18 M = 18 * 10? ² M
New answer posted
5 months agoContributor-Level 9
P (CO? ) = K? X (CO? )
X (CO? ) = P (CO? )/K? = 0.835 / (1.67 * 10³) = 0.5 * 10? ³
X (CO? ) = n (CO? )/ (n (CO? ) + n (H? O) ≈ n (CO? )/n (H? O) (since n (CO? ) << n (H? O)
n (H? O) in 0.9L = 900g/18gmol? ¹ = 50 mol
n (CO? ) = X (CO? ) * n (H? O) = 0.5 * 10? ³ * 50 = 25 * 10? ³ moles = 25 mmol
New answer posted
5 months agoContributor-Level 9
C? H? + 13/2 O? → 4CO? + 5H? O
1 mole C? H? (58 g) produces 5 mole H? O (90 g)
∴ 90 g H? O obtained from 58 g C? H?
∴ 72g H? O obtained from (58/90) * 72g = 46.4 g
= 464 * 10? ¹g
New answer posted
5 months agoContributor-Level 9
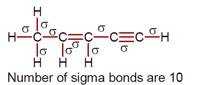
Number of sigma bonds are 10.
[Structure showing 10 sigma bonds in the molecule]
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers