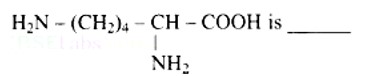Chemistry NCERT Exemplar Solutions Class 12th Chapter Fourteen
Get insights from 85 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Fourteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Fourteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoLysine,
(a) α - Amino acid
(b) Basic amino acid
(c) Amino acid synthesized in body
(d) β- Amino acid
Contributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct options are a, b and c.
Lysine is an amino acid with the structural formula of (a).
(b) Because the number of groups (two) is more than the number of -COOH groups (one), it is a basic amino acid (one).
Because it is created in human bodies, it is a non-essential amino acid.
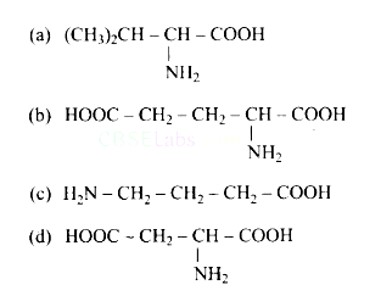
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct options are b and d.
Acidic amino acids are divided into two groups, one -COOH group against the other - NH2 group. As a result, the acidic amino acids (b) and (d) are the only ones among the supplied structures.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct options are a and c.
Globular protein is a protein structure that forms when a chain of polypeptides coils around to produce a spherical shape. Insulin and albumin are two examples of water-soluble globular proteins. As a result, (a) and (c) are the correct answers.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct options are b and d.
Sucrose is a common disaccharide that breaks down into an equimolar mixture of D- (+)- glucose and D- (-)- fructose when hydrolyzed. These two monosaccharides are held together by a glycosidic bond between C1 of α - glucose and C2 of β fructose. Because the reducing groups of glucose and fructose are involved in the formation of glycosidic linkages, sucrose is a non-reducing sugar.
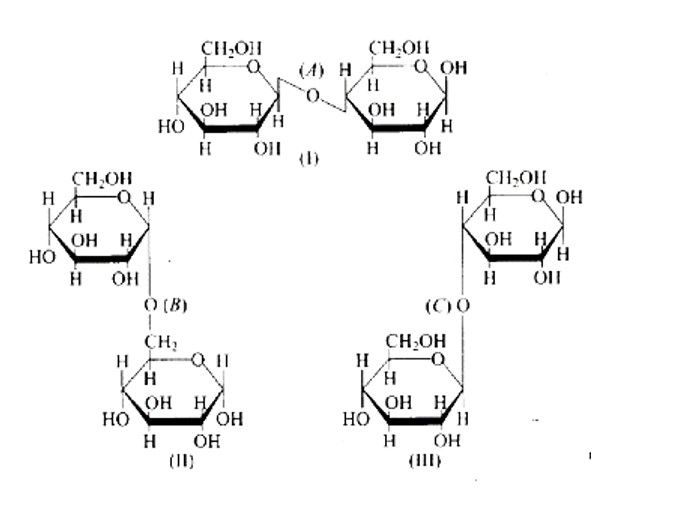
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Correct option is C.
The links between C1 and C4 of glucose are shown as 'A' and ' C, ' respectively, whilst the linkage between C1 and C6 of the glucose units is shown as ' B. ' Furthermore, option (c) is clearly correct based on the structures.
Because ' B ' is the bond or link between the glucose units of C1 and C6 and, option (b) is wrong.
Option (a) is wrong because the relationship between C1 and C4 and is represented by 'C.'
Because 'A' is the hyperlink between C1 and C4 the glucose units of and, option (d) is wrong.
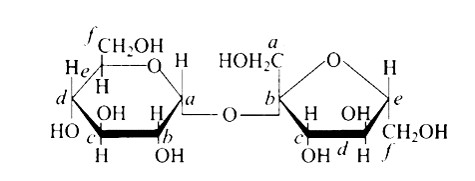
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is c.
In the cyclic structure of glucose or fructose, anomeric carbon is carbon that is close to an oxygen atom. As shown in the structure above, atoms 'a' and 'b' are close to the oxygen atom, and the hydroxyl groups of both carbon atoms have different orientations.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is a.
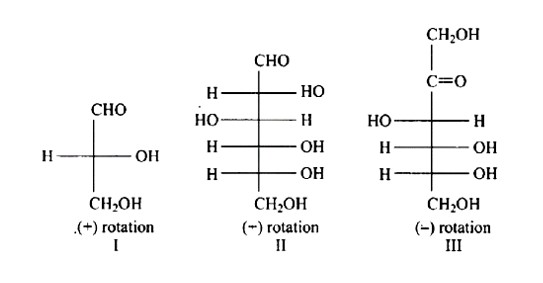
In the same way as (+) glyceraldehyde has a group on the lowest asymmetric carbon on the right side, the I, II, and III structures have a (-OH) group on the lowest asymmetric carbon on the right side giving them a D-configuration.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is c.
The absence of a free (-CHO) aldehyde group is indicated by the fact that glucose pentaacetate does not react with hydroxylamine. The open structure of glucose cannot account for this, although the open chain structure of glucose can account for all other features.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
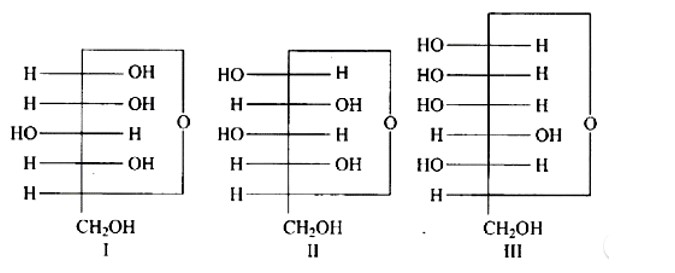
Ans: The Correct option is a.
Anomers are cyclic monosaccharide structures that differ structurally at carbon-1. In this scenario, I and II are anomers since they differ only at carbon -1.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is d.
Adenine, guanine, thymine, and cytosine are the four bases found in DNA. As a result, uracil is not found in DNA as we cannot identify it among the other nucleotides that are present in the DNA. Uracil is, however, seen in RNA, another nucleic acid found in most of the organisms.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers