Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
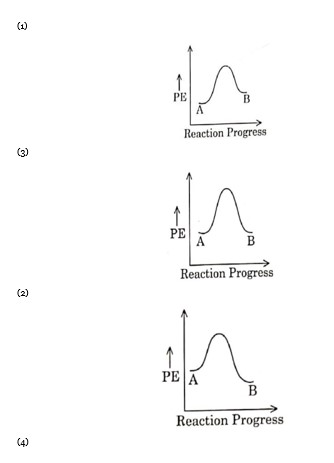
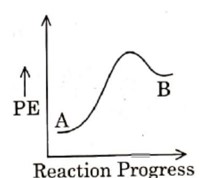
For a given reaction? H is negative. Hence, potential energy profile is of an exothermic reaction.
New answer posted
3 months agoContributor-Level 10
Due to lanthanoid contraction Zr and Hf has similar atomic and ionic radii.
New answer posted
3 months agoContributor-Level 10
No. of atoms in Hexagonal primitive unit cell = 6
No. of Tetrahedral voids = 2 * No. of atoms per unit cell
= 2 * 6 = 12
No. of Octahedral voids = No. of atoms per unit cell = 6
New answer posted
3 months agoBeginner-Level 5
The valence bond theory explains the covalent bond formation for two half-filled orbitals. The bond strength depends on several factors, including the extent of overlap of the two atomic orbitals. The bond strength is directly proportional to the extent of overlap.
In simple words, the greater the amount of overlap between two orbitals, the stronger the covalent bond will be.
JEE asks many questions based on the comparison of bond strength for two different pairs of atomic orbitals forming a covalent bond. For example, why H–F bond stronger than the F–F bond?
New answer posted
3 months agoContributor-Level 10
Pollution due to oxides of sulphur gets enhanced due to hydrocarbons and oxidizing agents like O? & H? O?
New answer posted
3 months agoContributor-Level 10
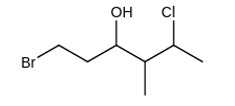
The IUPAC name giving the lowest number to the functional group obeying the lowest set of locants is 1-bromo-5-chloro-4-methylhexan-3-ol.
New answer posted
3 months agoContributor-Level 10
More the number of strong field ligands in a complex greater the energy absorbed by a complex.
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 10
M = (a³ * d * N_A) / Z = (3.608 * 10? )³ * 8.92 * 6.022 * 10²³) / 4
M = (46.96 * 10? ²? * 8.92 * 6.022 * 10²³) / 4 = 63 g/mole
the closest answer is choice (1)
P = (nRT) / V = (2 * 0.0831 * 300) / 10 = 4.986 bar
New question posted
3 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers