Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Moles of HCl = (50 / 1000) * 0.5 = 0.025 moles
So, moles of CaCO? used = 0.025 / 2 = 0.0125 moles = 1.25 g
95% (Total mass of CaCO? ) = 1.25 g
Total mass of CaCO? = 1.25 / 0.95 = 1.32 g
New answer posted
3 months agoContributor-Level 10
Thermosetting polymers such as Bakelite cannot be moulded again and are hence not reusable.
New answer posted
3 months agoContributor-Level 10
p? = p? x? is not a correct form of Dalton's law of partial pressures.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
For a zero-order reaction Rate Vs conc graph will be straight line parallel to the x-axis.
For a 1st order reaction t? /? vs concentration will again be a straight line parallel to x-axis.
New answer posted
3 months agoContributor-Level 10
At different positions -NO? affects acidic strength differently. The order of acidic strength is p-nitrophenol > o-nitrophenol > m-nitrophenol.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
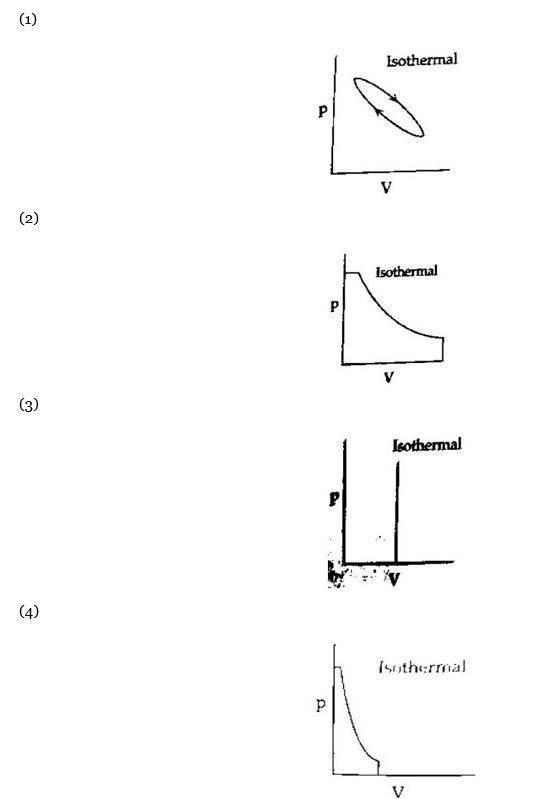
Maximum work is done in the case where area under the curve is maximum.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers