Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
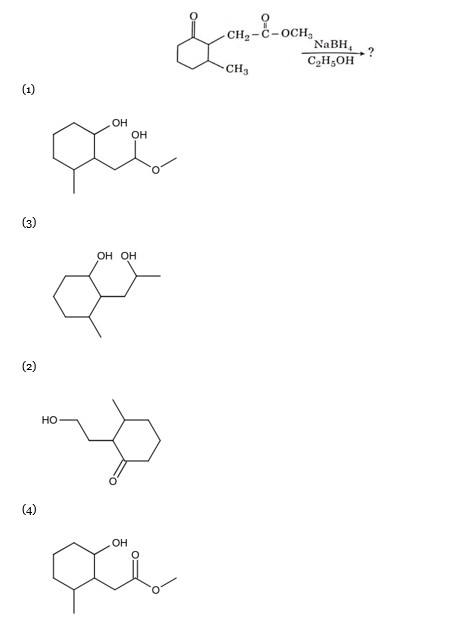
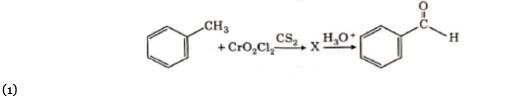
R: CH? OH
Certain mild reducing agents like hypophosphorus acid or ethanol reduce diazonium salts to arene and themselves get oxidised to phosphorous acid and ethanal respectively.
New answer posted
3 months agoContributor-Level 10
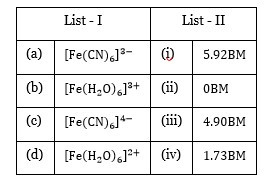
[Fe (CN)? ]? ³ Fe? ³ = 3d?
Unpaired electron = 1, μ = 1.7BM
[Fe (H? O)? ]? ³ Fe? ³ = 3d?
Unpaired electrons = 5, μ = 5.9BM
[Fe (CN)? ]? Fe? ² = 3d?
Unpaired electron = 0, μ = 0BM
[Fe (H? O)? ]? ² Fe? ² = 3d?
Unpaired electrons = 4, μ = 4.9BM
New answer posted
3 months agoContributor-Level 10
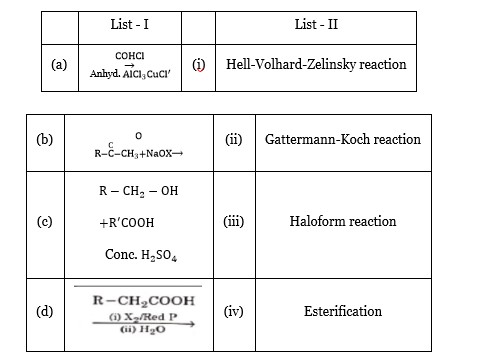
(a) List-I List-II
CO, HCl Anhydrous AlCl? /CuCl → (ii) Gattermann-Koch reaction
(b) R – C=O .
(c) R – CH? OH + R'COOH - (conc. H? SO? )-> (iv) Esterification
(d) R – CH? COOH - ( (i) X? /RedP (ii) H? O )-> (i) Hell-Volhard Zelinsky reaction
New answer posted
3 months agoContributor-Level 10
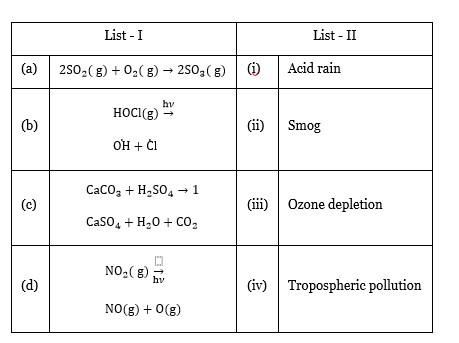
| List-I | | List-II |
| :- | :- | :- |
| (a) 2SO? (g) + O? (g) → 2SO? (g) | (iv) | Tropospheric pollution |
| (b) HOCl (g) - (hν)->? H +? l | (iii) | Ozone depletion |
| (c) CaCO? + H? SO? → | (i) CaSO? + H? O + CO? | Acid rain |
| (d) NO? (g) - (hν)-> NO (g) + O (g) | (ii) | Smog |
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
CH? – CH? – COO? Na? - (NaOH, CaO, Heat)-> CH? – CH? + Na? CO?
Decarboxylation takes place by soda-lime (NaOH + CaO)
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
Dihedral angle (D.A.) of least stable conformer of ethane = 0°
D.A. = 0°
(Eclipsed)
New answer posted
3 months agoContributor-Level 10
Most of the trivalent lanthanoid ions are coloured in the solid state.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers