Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Dumas method,
Moles of N in N, N-dimethylaminopentane (C7H17N)
= 11.25 mol
= 1125 * 10-2 mol
Ans. = 1125
New answer posted
5 months agoContributor-Level 10
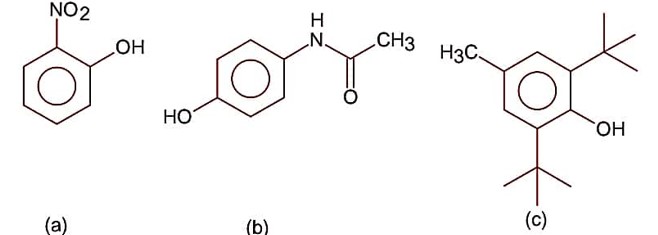
(a) Shows intra molecular H-bonding.
(b) Shows inter molecular H-bonding.
(c) It does not shows intermolecular H-bonding due to high steric hindrance at o-position of benzene ring.
New answer posted
5 months agoContributor-Level 10
mvr =
(Bohr's kinetic energy)
Comparing with
x = 32π2 = 315.50
10x = 3155
New answer posted
5 months agoContributor-Level 10
(1) Standard enthalpy of formation for alkali metal bromides becomes more negative on descending down the group.
(2) Standard enthalpy of formation for LiF is most negative among alkali metal fluorides.
(3) In case of Csl, lattice energy is less but Cs+ having less hydration energy due to which it is less soluble in water.
(4) For alkali metal fluorides, the solubility in water increases from Li to Cs. LiF is least soluble in water.
New answer posted
5 months agoContributor-Level 10
Let mass of water initially = x gram
Mass of sucrose = (1000 – x) gram
Mole of sucrose =
0.75 molal Þ 0.75 mole solute in 1 kg of solvent
a = 0.2775kg or 277.5 gram
Ice separated = (795.86 – 277.5) gram
= 518.3 gram
Ans. = 518 (the nearest integer)
New answer posted
5 months agoContributor-Level 10
In the lyophilic colloids, the colloidal particles are extremely solvated.
New answer posted
5 months agoContributor-Level 10
Ozone in the stratosphere is a product of UV radiations acting on O2 molecule.
New answer posted
5 months agoContributor-Level 10
Given that, rate of appearance of Cr2(SO4)3 =
LHS RHS
Rate of disappearance of C2H6O =
Rate of disappearance of C2H6O = 4.005 mol /min
Ans. = 4 (nearest integer)
New answer posted
5 months agoContributor-Level 10
Mass of organic compound = 0.2 gram
= 0.001 mol
Mass of Br = 0.001 * 80g
= 0.08 g
Mass & of Br =
= 40 %
New answer posted
5 months agoContributor-Level 10
Mn6+ has one unpaired electron so paramagnetic and has green colour.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers