Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 9
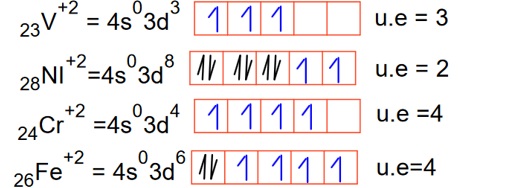
the lowest spin only magnetic moment for Ni+2 because of minimum unpaired electron.
New answer posted
5 months agoContributor-Level 10
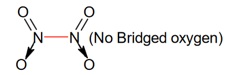
For AB3 interhalogen compound, which is T-shaped, only two lone pair electrons are available on central atom.
New answer posted
5 months agoContributor-Level 10
Except electron gain enthalpy all the physical properties like sublimation enthalpy, ionization enthalpy and hydration energy affected the value of reduction potential.
Ans. = 3
New answer posted
5 months agoContributor-Level 10
Electronic configuration of
Gd is ; Gd64 = [Xe]4f7 5d1 6s2
Hence
Total number of electrons in 4f sub-shell = 7
Ans. = 7
New answer posted
5 months agoContributor-Level 10
Molecular structure of tetrapeptide Gly-Glu-Asp-Tyr is;
Total negative charge developed at pH 12.5 = 4
Ans. = 4
New answer posted
5 months agoContributor-Level 10
KCl solution has molality (m) = 3.3, [Means 3.3 mol of KCl dissolved in 1 kg of solved]
Total mass of solution = mass of solute + mass of solvent
= 3.3 * 74.5 + 1000 gm
= 1245.85 gm
Volume of solution =
Ans. = 3 (the nearest integer)
New answer posted
5 months agoContributor-Level 10
0.0504 M NH4Cl of 5ml => millimole of
0.0210 M NH3 of 2ml => millimole of NH3 = 0.0210 * 2
It is a basic buffer.
Total volume = 7ml
Ans. = 3
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers