Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
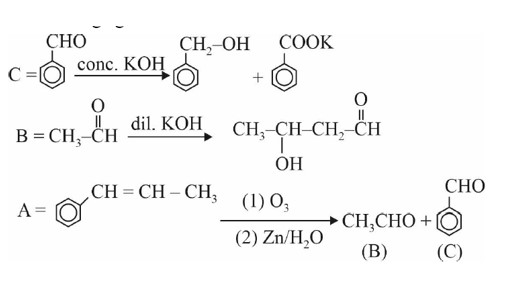
As the difference in and ionisation energy is high so atom contains 3 valence electrons. Hence, valency of the atom is 3 and the metal is trivalent. 4 mole of trivalent metal hydroxide will react with 6 mole of ,
New answer posted
3 months agoContributor-Level 9
Photochemical smog has high concentration of oxidising agent like and and is therefore called as oxidising agent.
New answer posted
3 months agoContributor-Level 9
- atom
or
or
Hence, number of different lines possible
Minimally, both can have same transition i.e etc.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
It's the energy that is needed for a change of state to transition from one substance or physical state to another. Remember that the main condition for latent heat is that there is no temperature change when the energy is absorbed or released.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers